PRACE INSTYTUTU ODLEWNICTWA
|
|
|
- Marian Jaworski
- 8 lat temu
- Przeglądów:
Transkrypt
1 PRACE INTYTUTU ODLEWNICTWA Tom LII Rok 212 Zeszyt 3 DOI: 1.736/iod INFLUENCE OF CHEMICAL MODIFICATION OF THE OLUBLE ODIUM ILICATE ON THE ZETA POTENTIAL OF THE COLLOIDAL PARTICLE IN A OLUBLE ODIUM ILICATE ETER HARDENER GELLING YTEM WPŁYW MODYFIKACJI CHEMICZNEJ ROZPUZCZALNEGO KRZEMIANU ODU NA POTENCJAŁ ZETA CZĄTECZEK KOLOIDALNYCH W UKŁADZIE ŻELUJĄCYM ROZPUZCZALNY KRZEMIAN ODU UTWARDZACZ ETROWY Andrzej Baliński Instytut Odlewnictwa, ul. Zakopiańska 73, Kraków Abstract The article presents how to perform a chemical modification of the soluble sodium silicate with morphoactive, organic compounds. An attempt was made to determine the effect of chemical modifiers of the soluble sodium silicate on its physicochemical and structural properties. The kinetics of changes in the Zeta potential of a "chemically modified soluble sodium silicate ester hardener" gelling system was described. Key words: soluble sodium silicate, chemical modification, Zeta potential treszczenie W artykule przedstawiono sposób modyfikacji chemicznej uwodnionego krzemianu sodu za pomocą morfoaktywnych związków organicznych. Podjęto próbę określenia wpływu modyfikatorów chemicznych uwodnionego krzemianu sodu na jego właściwości fizykochemiczne i strukturalne. Opisano kinetykę zmian wartości potencjału Zeta żelującego układu modyfikowany chemicznie uwodniony krzemian sodu. łowa kluczowe: uwodniony krzemian sodu, modyfikacja chemiczna, potencjał Zeta Introduction oluble sodium silicate having the general formula xna 2 O yio 2 is one of the most common inorganic binders. In the foundry industry, soluble sodium silicate is a binder used to make sand moulds and cores for castings from the ferrous and nonferrous metals. In the European countries, the share of castings made in these sands, hardened with liquid esters or carbon dioxide, is up to 3% of the total production. The advantage of moulding sands with the soluble sodium silicate is high heat resistance of moulds and cores, which is particularly important for medium and heavy castings, and also absence
2 A. Baliński of the emission of toxic gases during mould making and pouring. In addition to these environmental and technological advantages, there are also important economic considerations. Moulding sands with the soluble sodium silicate are much cheaper than their counterparts prepared with resin binders. However, compared to theresin-bonded moulding sands, besides some obvious advantages, moulding sands with the soluble sodium silicate have also several disadvantages, to mention only the excessively high residual strength, and hence the inferior knocking out properties and difficult reclamation of the used sand, as well as the low initial strength (especially cohesive). Another disadvantage of this type of moulding sand is also the formation of sinters resulting from the tendency of Na 2 O - io 2 to react with quartz sand at elevated temperatures. The sintering process can be reduced by reducing the amount of binder in the moulding sand and by suitable modification of the binder sand grain interface. Reducing the amount of silicate binder is possible only if its binding properties can be improved. It is the fact well-known that the binding properties of organic binders are improved due to the presence of the functional groups like -NH 2, -COOH, -CONH 2 and others [1 3]. It was recognized that the properties of the soluble sodium silicate can be improved by the introduction of modifiers containing these functional groups. The used modifiers tend to form with the soluble sodium silicate an IPN (Interpenetrating Polymer Networks) type network, which is a mutually interpenetrating polymer network. The consequence is the reduced final strength of moulding sand produced with the modified solution of soluble silicate. Regardless of the influence of the IPN network, the strength properties of silica gel are also affected by the characteristics of its structure associated with the hardening kinetics, depending on the kinetics of changes in the Zeta potential of the particles in a "soluble sodium silicate ester hardener" gelling system. Methodology of measurements Chemical modification of soluble sodium silicate was performed with the organic morphoactive compounds C1, C3 and C6. The chemical modification consisted in introducing to an autoclave the sodium silicate glaze with the modulus M = 3.1, and demineralised water. The autoclaving process was carried out under constant conditions, i.e. at a temperature of 16 C to 18 C and a pressure from.6 MPa to.8 MPa. To obtain the required amount of the soluble sodium silicate, for the operation of concentration and to confirm the reproducibility of the results, several dissolution tests were performed on the sodium silicate glaze. The duration of the dissolution process was approximately 3 hours. The solution in the autoclave was decompressed, and then the autoclave was evacuated. The soluble sodium silicate after the autoclaving process and concentration was subjected to filtration. The soluble sodium silicate had a density of g/cm 3 and molar modulus of about 3.. This solution was subjected to correction of the modulus M to about 2. and was concentrated to a density of about 1. g/cm 3. Chemical modification was carried out before the concentration step, introducing to the soluble sodium silicate solution with a corrected modulus, the morphologically active agents C1, C3 and C6, added in an amount of.1%,.% and 1.%, calculated in relation to the total amount of oxides 6 Prace IOd 3/212
3 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of... present in the soluble sodium silicate (Na 2 O + io 2 ). The characteristics of the obtained types of the sodium silicate are shown in Table 1. Table 1. Characteristics of the basic physico-chemical properties of chemically modified types of the soluble sodium silicate and of a standard chemically non-modified soluble sodium silicate () Tabela 1. Charakterystyka podstawowych właściwości fizykochemicznych modyfikowanych chemicznie rodzajów uwodnionego krzemianu sodu oraz wzorcowego, niemodyfikowanego chemicznie uwodnionego krzemianu sodu () Type of soluble sodium silicate Type of modifier Modifier content, wt. % Oxides content, wt. % Na 2 O io 2 Modulus, M Density, g/cm 3 C1-.1 C C1-. C C1-1. C C3-.1 C C3-. C C3-1. C C6-.1 C C6-. C C6-1. C Under the effect of water, the surface of silicon dioxide and of other insoluble oxides undergoes the process of hydroxylation. The formation of hydroxyl groups depends on the physico-chemical conditions. On the surface of io 2 there are surface hydroxyl groups (silanols) and, in some cases, water molecules inside the silica particles. The properties of such silica depend on the geometrical structure of pores and on the chemical properties of the silica surface [4, ]. The skeleton of silica gel consists of spherical particles joined at points of contact. The pores are voids between the particles with different degree of packing. The size of the particles determines the size of the specific surface, and the density of packing determines the volume of pores and their radii. The silanol groups (i OH) present on the silica surface are formed by two main processes. uch groups are formed in the synthesis of silica, and more specifically in the polymerisation of i(oh) 4. The supersaturated solution of silicic acid enters the polymerised form, which is next transformed into spherical colloidal particles containing surface groups i OH. During drying, hydrogel is transformed into the final product xerogel, which contains some or most of the surface silanol groups. The i OH surface groups can also form in water or in an aqueous solution as a result of the rehydroxylation of silica free from the hydroxyl groups. The surface silicon atoms tend to form a tetrahedron configuration and in aqueous solutions their free bonds are saturable with hydroxyl groups [4, ]. Prace IOd 3/212 7
4 A. Baliński The electric charge on the surface of the metal oxide in an aqueous electrolyte solution is formed due to a reaction of the surface hydroxyl groups with ions from the solution. The reaction of these groups with the H + or OH - ions in an electrolyte solution leads to the formation of groups endowed with positive or negative charge. These reactions are set out in the theory of site binding as ionisation reactions of the surface hydroxyl groups [6 1] OH OH H 2 (1) + OH O + H (2) where is the surface. In view of the fact that H + ions create a charge on the surface of oxides, in these systems they are considered the potential-forming ions. An important role in the formation of charge for such systems play the ions in a carrier electrolyte. Early classic theories of the electrical double layer (edl) initially assumed that these ions are subject to nonspecific adsorption, while in more complex models of the edl, ions of the carrier electrolyte can also undergo the specific adsorption. According to the theory of "site binding", characteristic of these systems, carrier electrolyte ions react with the oxide surface hydroxyl groups to form a complex bond, e.g. for the metal oxide with a hypothetical electrolyte AnCt acting as a carrier electrolyte. The following complex reactions may occur on the surface of oxide: OH An OH + An + H (3) + OH+Ct O Ct + H (4) where Ct + represents a cation and An - anion of the carrier electrolyte. The mechanism of the charge formation on the surface of metal oxides in electrolyte solutions based on the reactions of 1 4 allows for recognition of the observed in these systems increase in charge density and the simultaneous decrease in Zeta potential with increasing concentration of the electrolyte [6, 11 14]. The reactions of ionisation and complexation of surface hydroxyl groups can be described with the following thermodynamic equilibrium constants: exp () exp (6) 8 Prace IOd 3/212
5 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of... (7) (8) were: K a1 the equilibrium dissociation constant for the OH + group, 2 K a2 the equilibrium constant for the formation of the O - surface groups, Ψ T k e surface potential, temperature, Boltzmann constant, elementary charge (electron), γ H the activity coefficient of H + ions, γ the activity coefficient of OH groups, γ + the activity coefficient of OH + 2 group, γ - the activity coefficient of O - groups, K An the equilibrium constant for the reaction of anion complexation, K Ct the equilibrium constant for the reaction of the cation complexation, Ψ ß the potential of inner Helmholtz plane, γ An the activity coefficient of anions An _, γ Ct the activity coefficient of cations Ct +, γ ± + the activity coefficient OH2 An groups, + γ the activity coefficient O Ct groups. The equilibrium constants of the ionisation and complexation reactions are not only the parameters characterising the electrical double layer at the metal oxide aqueous electrolyte solution interface, but also allow us to calculate the charge and potential distribution within the edl. Our knowledge of the distribution of the electric potential and electric charge within the edl is one of the important elements in the theory of the stability of dispersed systems. Moreover, these constants, as well as the complexation constants in solution, allow us to calculate the concentration of each surface compound, and in the case of ions adsorption the ion partition coefficient between the solution and solid phase. Prace IOd 3/212 9
6 A. Baliński The surface charge density is proportional to an algebraic sum of the concentration of the ionised groups and complexes: σ {[ OH ] + [ OH An ] [ O ] [ O ]} = Ct B N 2 2 where Ct + represents a cation and An - an anion of the carrier electrolyte. The concentration of the potential-forming ions at which the surface charge density is zero is called point of zero-charge (pzc). In the case of metal oxide, the potentialforming ions are H + ions. Therefore, this point is related with a ph scale and is referred to as ph pzc. In ph pzc the total charge density is zero, which means that the concentration of positively charged groups is equal to the concentration of negatively charged groups: [ ] + [ OH An ] = [ O ] + [ O ] σ = OH 2 2 Ct The carrier electrolyte ions involved in reactions 3 and 4 occupy positions in the "inner Helmholtz plane" (IHP) and are labelled with the β symbol. This level is attributed to the specifically adsorbed ions σ β and potential ψ β. The density of charge adsorbed by the IHP in a "metal oxide - electrolyte solution" system (1:1) is equal to: (9) (1) σ β + + {[ O Ct ] [ OH ]} = An B N 2 (11) To estimate the density of ions adsorbed by the IHP it is assumed that ion density in the carrier electrolyte located in the edl diffusion layer corresponds to the electric charge in the diffusion layer and can be calculated from the Gouy-Chapman equation, knowing the value of Zeta potential. Thus, the density of ions forming surface complexes is equal to: σ β, An( Ct ) = σ An( ct) σ d (12) Part of the surface charge derived from the ionised groups is compensated in the diffusion part of the electrical double layer. The diffusion part of the layer accumulates ions which are adsorbed by an electrostatic interaction. The adsorption of these ions is called non-specific adsorption. The diffusion layer is characterised by the diffusion layer potential ψ d and diffusion layer charge density σ. The charge density of the diffusion d layer will be equal to: + {[ O ] [ OH ]} σ d = B N 2 (13) The relationship between surface potential and charge density for a symmetric electrolyte is expressed by the equation: σ d = zeψ d 8εε crt sinh kt (14) 1 Prace IOd 3/212
7 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of... where: ψ d the potential of diffusion layer, T temperature, k Boltzmann constant, e elementary charge (electron), z valence ions of the carrier electrolyte, ε relative dielectric permittivity of the medium, ε dielectric permittivity of vacuum, R gas constant, c concentration of the electrolyte. The potential of the diffusion layer of the electrical double layer edl can be determined from the measurements of Zeta potential, allowing for a distance between the slip plane (determining the value of Zeta potential) and the "outer Helmholtz plane" (OHP), with which the potential of the diffusion layer is associated. To determine the value of the diffusion layer potential it is necessary to know the slip plane distance from the OHP. This distance is not determined in a consistent manner and depending on the system ranges from.4 nm to 2. nm. It depends, among others, on the porosity of the solid [1]. As stated above, ion concentration at which the Zeta potential is zero is called "isoelectric point". In the case of metal oxides, this point is also referred to the ph scale. At the isoelectric point, the charge density in the diffusion layer is equal to. This means that on the oxide surface the concentration of positively ionised groups is equal to the concentration of negatively ionised groups. The Zeta potential of colloids with the liquid phase acting as a dispersed phase is usually determined by microelectrophoresis. Other electrokinetic phenomena are much less common in such cases [16 19]. The correct values of electrophoretic mobility (free from the so-called side wall effect ), at the stationary level in zeta meters with a constant voltage, are obtained for a rectangular cell with a height-to-width ratio greater than 1:2. The hydroxyl groups on the surface of io 2 in electrolyte solutions behave similarly to hydroxyl groups on the surface of metal oxides. The charge on the surface is formed by the reaction of surface hydroxyl groups (silanol groups) with the ions H + or OH - and by complexation reactions with ions of the carrier electrolyte. To determine changes in the Zeta potential after certain time of gelation going on in the "soluble sodium silicate ester hardener" system, a sample was taken and introduced to the carrier electrolyte. Before making the dispersion, the electrolyte solutions were filtered on a membrane filter with the pore diameter of.22 µm to remove the insoluble impurities. Based on the results of the preliminary measurements, an optimum ratio of the "soluble sodium silicate ester hardener" sample addition to the carrier electrolyte was determined. With this ratio, the signal recorded for the scattered laser light was optimal, and the measured values of Zeta potential were the same as at a higher dilution. After Prace IOd 3/212 11
8 A. Baliński preparation, the dispersed sample was subjected to the effect of ultrasounds and its ph value was measured. ince the systems studied were characterised by certain heterogeneity of the dispersed phase, the average Zeta potential distribution was calculated by averaging the individual distributions (summed channel by channel, and calculating the average of 3 measurements). Preliminary measurements of the Zeta potential of the examined "soluble sodium silicate ester hardener" samples revealed the presence of particles undergoing electrophoresis. The measurement results are shown in Figure C1-.1 C1-. C1-1. C3-.1 C3-. C3-1. C6-.1 C6-. C Kind of soluble sodium silicate Fig. 1. Zeta potential of the investigated types of soluble sodium silicate Rys. 1. Potencjał Zeta badanych rodzajów uwodnionego krzemianu sodu The study of the kinetics of changes in the electrokinetic Zeta potential in function of the gelation time was carried out on the "soluble sodium silicate hardener ester" gel samples taken after the gelation time of 2, 3, 4, 6, 8 and 1 min. Figures 2 19 show changes in the Zeta potential of gel samples obtained from the following soluble sodium silicates: C1-.1, C1-., C1-1., C3-.1, C3-., C3-1., C6-.1, C6-., C6-1., and from the chemically unmodified standard soluble silicates. The ph values of dispersion samples obtained from the gel varied from ph 11 for the shortest gelation time to ph 1.3 obtained after 1 minutes of gelation. The ph values differed little for for individual samples and had no significant effect on the Zeta potential values. Figures 2 2 show the value of the first maximum of electrophoretic signal intensity / on the surface of colloidal particles in the gelling system "chemically modified and unmodified soluble sodium silicate ester hardener" gelling system and the corresponding Zeta potential values. Figures show the value of the second maximum of electrophoretic signal intensity I on the surface of colloidal particles in the "chemically modified and unmodified soluble sodium silicate ester hardener" gelling system and the corresponding Zeta potential values. 12 Prace IOd 3/212
9 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of C C1-. C Fig. 2. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 2 minutes the "soluble sodium silicate of type C1 and ester hardener" system Rys. 2. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 2 minut układu uwodniony krzemian typu C1 i 3 2 C C C Fig. 3. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 2 minutes the "soluble sodium silicate of type C3 and ester hardener" system Rys. 3. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 2 minut układu uwodniony krzemian typu C3 i Prace IOd 3/212 13
10 A. Baliński 3 2 C C6-. C Fig. 4. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 2 minutes the "soluble sodium silicate of type C6 and ester hardener" system Rys. 4. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 2 minut układu uwodniony krzemian typu C6 i 3 2 C C C Fig.. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 3 minutes the "soluble sodium silicate of type C1 and ester hardener" system Rys.. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 3 minut układu uwodniony krzemian typu C1 i 14 Prace IOd 3/212
11 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of C C C Fig. 6. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 3 minutes the "soluble sodium silicate of type C3 and ester hardener" system Rys. 6. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 3 minut układu uwodniony krzemian typu C3 i 3 2 C C6-. C Fig. 7. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 3 minutes of the "soluble sodium silicate of type C6 and ester hardener" system Rys. 7. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 3 minut układu uwodniony krzemian typu C6 i Prace IOd 3/212 1
12 A. Baliński C1-.1 C1-. C Fig. 8. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 4 minutes the "soluble sodium silicate of type C1 and ester hardener" system Rys. 8. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 4 minut układu uwodniony krzemian typu C1 i 3 2 C C C Fig. 9. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 4 minutes the "soluble sodium silicate of type C3 and ester hardener" system Rys. 9. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 4 minut układu uwodniony krzemian typu C3 i 16 Prace IOd 3/212
13 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of C C C Fig. 1. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 4 minutes the "soluble sodium silicate of type C6 and ester hardener" system Rys. 1. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 4 minut układu uwodniony krzemian typu C6 i 3 2 C C1-. 1 C Fig. 11. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 6 minutes the "soluble sodium silicate of type C1 and ester hardener" system Rys. 11. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 6 minut układu uwodniony krzemian typu C1 i Prace IOd 3/212 17
14 A. Baliński 3 2 C C3-. C Fig. 12. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 6 minutes the "soluble sodium silicate of type C3 and ester hardener" system Rys. 12. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 6 minut układu uwodniony krzemian typu C3 i 3 2 C C C Fig. 13. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 6 minutes the "soluble sodium silicate of type C6 and ester hardener" system Rys. 13. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 6 minut układu uwodniony krzemian typu C6 i 18 Prace IOd 3/212
15 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of C C C Fig. 14. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 8 minutes the "soluble sodium silicate of type C1 and ester hardener" system Rys. 14. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 8 minut układu uwodniony krzemian typu C1 i 3 2 C C C Fig. 1. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after 8 minutes the "soluble sodium silicate of type C3 and ester hardener" system Rys. 1. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 8 minut układu uwodniony krzemian typu C3 i Prace IOd 3/212 19
16 A. Baliński 3 2 C C C Zeta potential, % Fig. 16. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 8 minutes the "soluble sodium silicate of type C6 and ester hardener" system Rys. 16. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 8 minut układu uwodniony krzemian typu C6 i C1-.1 C1-. C Fig. 17. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 1 minutes the "soluble sodium silicate of type C1 and ester hardener" system Rys. 17. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 1 minut układu uwodniony krzemian typu C1 i 2 Prace IOd 3/212
17 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of C C C Fig. 18. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 1 minutes the "soluble sodium silicate of type C3 and ester hardener" system Rys. 18. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 1 minut układu uwodniony krzemian typu C3 i C6-.1 C6-. C Fig. 19. Electrophoretic signal intensity I as a function of Zeta potential of colloidal particles formed after gelling for 1 minutes the "soluble sodium silicate of type C6 and ester hardener" system Rys. 19. Intensywność sygnału elektroforetycznego I w funkcji potencjału Zeta cząstek koloidalnych powstałych po czasie żelowania 1 minut układu uwodniony krzemian typu C6 i Prace IOd 3/212 21
18 A. Baliński Gelation time, min C1-.1 C1-. C1-1. Fig. 2. The first maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C1-.1, C1-., C1-1. and ester hardener" system Rys. 2. Pierwsze maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C1-,1, C1-,, C1-1, i Gelation time, min C3-.1 C3-. C3-1. Fig. 21. The first maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C3-.1, C3-., C3-1. and ester hardener" system Rys. 21. Pierwsze maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C3-,1, C3-,, C3-1, i 22 Prace IOd 3/212
19 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of Gelation time, min C6-.1 C6-. C6-1. Fig. 22. The first maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C6-.1, C6-., C6-1. and ester hardener" system Rys. 22. Pierwsze maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C6-,1, C6-,, C6-1, i Gelation time, min C1-.1 C3-.1 C6-.1 Fig. 23. The first maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C1-.1, C3-.1, C6-.1 and ester hardener" system Rys. 23. Pierwsze maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C1-,1, C3-,1, C6-,1 i Prace IOd 3/212 23
20 A. Baliński Gelation time, min C1-. C3-. C6-. Fig. 24. The first maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C1-., C3-., C6-. and ester hardener" system Rys. 24. Pierwsze maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C1-,, C3-,, C6-, i Gelation time, min C1-1. C3-1. C6-1. Fig. 2. The first maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C1-1., C3-1., C6-1. and ester hardener" system Rys. 2. Pierwsze maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C1-1,, C3-1,, C6-1, i 24 Prace IOd 3/212
21 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of Gelation time, min C1-.1 C1-. C1-1. Fig. 26. The second maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C1-.1, C1-., C1-1. and ester hardener" system Rys. 26. Drugie maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C1-,1, C1-,, C1-1, i Gelation time, min C3-.1 C3-. C3-1. Fig. 27. The second maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C3-.1, C3-., C3-1. and ester hardener" system Rys. 27. Drugie maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C3-,1, C3-,, C3-1, i Prace IOd 3/212 2
22 A. Baliński Gelation time, min C6-.1 C6-. C6-1. Fig. 28. The second maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C6-.1, C6-., C6-1. and ester hardener" system Rys. 28. Drugie maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C6-,1, C6-,, C6-1, i C1-.1 C3-.1 C6-.1 Gelation time, min Fig. 29. The second maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C1-.1, C3-.1, C6-.1 and ester hardener" system Rys. 29. Drugie maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C1-.1, C3-.1, C6-.1 and ester hardener" 26 Prace IOd 3/212
23 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of Gelation time, min C1-. C3-. C6-. Fig. 3. The second maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C1-., C3-., C6-. and ester hardener" system Rys. 3. Drugie maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C1-,, C3-,, C6-, i Gelation time, min C1-1. C3-1. C6-1. Fig. 31. The second maximum of electrophoretic signal intensity I and corresponding Zeta potential of colloidal particles formed after gelling the "soluble sodium silicate type C1-1., C3-1., C6-1. and ester hardener" system Rys. 31. Drugie maksimum intensywności sygnału elektroforetycznego I oraz odpowiadający mu potencjał Zeta cząstek koloidalnych powstających w żelującym układzie uwodniony krzemian sodu rodzaju C1-1,, C3-1,, C6-1, i Prace IOd 3/212 27
24 A. Baliński Results and discussion For the standard, chemically non-modified soluble sodium silicate, the change in the Zeta potential of particles in a "chemically unmodified soluble sodium silicate ester hardener" gelling system is different, compared to changes in the Zeta potential of particles in a "chemically modified soluble sodium silicate ester hardener" gelling system. The Zeta potential of particles in a "chemically unmodified soluble sodium silicate ester hardener" gelling system has a very low and virtually constant value (about - 9 mv) for the gelation time from 2 minutes to 6 minutes. After this time of gelation, the stability of the gelling system decreases strongly and continuously. For the majority of the investigated types of the chemically modified soluble sodium silicate there are two maxima of electrophoretic signal intensity I on the surface of colloidal particles formed in a gelation process of the "chemically modified soluble sodium silicate ester hardener" system. A higher value of the first maximum of intensity I refers almost entirely to the colloidal particles with the lowest value of Zeta potential (from about - 2 mv to about -1 mv), as compared to the value of the Zeta potential of particles in the second maximum with lower value electrophoretic signal intensity I. The distribution of electrophoretic signal intensity I on the surface of colloidal particles formed in a "chemically modified soluble sodium silicate ester hardener" system and the corresponding value of Zeta potential indicate different stability of the system in function of the gelation time and the amount of chemical modifier introduced. With increasing content of modifier C1, the Zeta potential of the particles responsible for the maximum electrophoretic signal intensity I was increasing. This type of modifier confers high stability to the system after the gelation time of from about 2 minutes to about 3 minutes. In the interval of about 3 minutes to about 6 minutes of the gelling time, the stability of the system is declining. After the gelling time from about 6 minutes to about 1 minutes, the stability of the system recurs once again. With application of modifier C3, high stability of the system was also reported after the gelling time from about 2 minutes to about 4 minutes. After this gelling time the stability of the system was decreasing, to increase once again after the gelling time of about 8 minutes. Modifier C6 confers high stability to the system after the gelling time from about 2 minutes to about 4 minutes. After the gelling time from about 4 minutes to about 6 minutes, a decrease in the system stability has been observed. The stability of the system has increased once again after the gelling time from about 6 minutes to about 1 minutes. Conclusions 1. An increase in the content of modifiers C1, C3 and C6 will confer more stability to the "chemically modified soluble sodium silicate ester hardener" system, with the prolonged time of gelation. For the modifier content of.1%,.% and 1.%, the Zeta potential of the particles is from about -7 mv to about -9 mv, from about -6 mv to about -9 mv, and from about -4 mv to about -9 mv, respectively, with the gelation time of from about 2 minutes to about 3 minutes, from about 2 minutes 28 Prace IOd 3/212
25 Influence of chemical modification of the soluble sodium silicate on the Zeta potential of... to about 4 minutes, and from about 2 minutes to about 6 minutes. At the same time, the maximum value of the Zeta potential decreases for the above mentioned gelation times of the system. 2. The smallest differences in the stability of the "chemically modified soluble sodium silicate ester hardener" system for the whole range of the gelation time are provided by modifier C3-.1 (from about -9 mv to about -6 mv) and by modifier C6-1. (from about -7 mv to about - mv). 3. All kinds of modifiers, i.e. C1, C3 and C6, confer increased stability to the "chemically modified soluble sodium silicate ester hardener" system after the gelation time of from about 8 minutes to about 1 minutes. Acknowledgement The results presented in this publication were based on a research conducted under the Target Project No. 6 T9 23/C.6137 "tarting the production of an environment- -friendly inorganic binder of new generation for casting of ferrous and nonferrous metal alloys". References 1. Baliński A., techman M., Różycka D.: Influence of modification of the soluble sodium silicate with morphoactive agents on the mechanical properties of the moulding sands in temperatures to 9 C. Materials Engineering, 23, Vol. X, No. 3, pp techman M., Różycka D., Baliński A.: Modification of Aqueous odium ilicate olutions with Morphoactive Agents. Kongres Te-Chem, zczecin, techman M., Różycka D., Baliński A.: Modification of Aqueous odium ilicate olutions with Morphoactive Agents. Polish Journal of Chemical Technology, 23, Vol., No. 3, pp Iler R.K.: The Chemistry of ilica olubility. Polymerization. Colloid and urface Properties and Biochemistry. Wiley-Intercience, New York, Kisielev A.V. et al: urface Chemical Compounds and their Role in Adsorption. Moscow tate University Press, Moscow, James R.O., Parks G.A.: Characterization of aqueous colloids by their electrical double-layer and intrinsic surface chemical properties. [In:] urface and Colloid ci., New York, Wiley Interscience, Baliński A., Janusz W.: Potencjał ζ uwodnionego krzemianu sodu. Archives of Foundry, 24, Vol. 4, No. 11, pp Baliński A., Janusz W.: Changes of potential ζ in a system of hydrated sodium silicate ethylene glycol. Advances In Technology of Machines and Mechanical Equipment, 22, Vol. 26, No. 4, pp Janusz W.: Electrical double layer at the metal oxide/electrolyte interface. [In:] J.P. Hsu, Interfacial Forces and Fields, Theory and application. urfactant ci., Vol. 8, Chapter 4, New York, Prace IOd 3/212 29
26 A. Baliński 1. Paszkiewicz M., Pałkowski W.: Potencjał ξ. Wyznaczanie punktu IPE ćwiczenie. Zakład Radiochemii i Chemii Koloidów UMC, Westall J, Hohl H.: A comparison of electrostatic model for the oxide/solution interface. Adv Colloid Interface ci., 198, No. 12, pp James R.O.: urface ionization and complexation at the colloid/aqueous electrolyte interface. [In:] Adsorption of Inorganic at olid/liquid Interfaces. Ann Arbor, MI: Ann Arbor ci., 1981, pp Yates D.E., Levine., Healy T.W.: ite-binding Model of the Electrical Double Layer at the Oxide/Water Interface. J. Chem. oc. Faraday Trans., 1974, Vol. 7, No Davis J.A., James R.O., Leckie J.O.: urface Ionization and Complexation at the Oxide/ Water Interface. I. Computation of Electrical Double Layer Properties in imple Electrolytes. J. Colloid Interface ci., 1978, Vol. 63, No Kallay N., prycha R., Tomić M., Žalac., Torbić Ž.: ome controversies in the understanding of equilibria in electrical interfacial layer. Croatica Chemica Acta, 199, No. 63, pp Hunter R.O.: Zeta Potential in Colloid cience. Academic Press, London, Minor M., Van der Linde A.J., Van Leeuwen H.P., Lyklema J.: Dynamic Aspects of Electrophoresis and Electroosomosis: A New Fast Method for Measuring Particle Mobilities. J. Colloid Interface ci., 1997, No. 189, pp Kosmulski M., Hartikainen J., Maczka E., Janusz W., Rosenholm J.B.: Multiinstrument tudy of the Electrophoretic Mobility of Fumed ilica. Analytical Chem., 22, No. 74, pp Parks G.A.: The Isoelectric Point of olid Oxides, olid Hydroxides, and Aqueous Hydroxo Complex ystems. Chem. Rev., 196, No. 6, pp Prace IOd 3/212
DUAL SIMILARITY OF VOLTAGE TO CURRENT AND CURRENT TO VOLTAGE TRANSFER FUNCTION OF HYBRID ACTIVE TWO- PORTS WITH CONVERSION
 ELEKTRYKA 0 Zeszyt (9) Rok LX Andrzej KUKIEŁKA Politechnika Śląska w Gliwicach DUAL SIMILARITY OF VOLTAGE TO CURRENT AND CURRENT TO VOLTAGE TRANSFER FUNCTION OF HYBRID ACTIVE TWO- PORTS WITH CONVERSION
ELEKTRYKA 0 Zeszyt (9) Rok LX Andrzej KUKIEŁKA Politechnika Śląska w Gliwicach DUAL SIMILARITY OF VOLTAGE TO CURRENT AND CURRENT TO VOLTAGE TRANSFER FUNCTION OF HYBRID ACTIVE TWO- PORTS WITH CONVERSION
PRACE INSTYTUTU ODLEWNICTWA TRANSACTIONS OF FOUNDRY RESEARCH INSTITUTE
 PRACE INSTYTUTU ODLEWNICTWA TRANSACTIONS OF FOUNDRY RESEARCH INSTITUTE Volume LV Year 2015 Number 1 2015 Instytut Odlewnictwa. All rights reserved. DOI: 10.7356/iod.2015.01 Wpływ wskaźnika struktury WB
PRACE INSTYTUTU ODLEWNICTWA TRANSACTIONS OF FOUNDRY RESEARCH INSTITUTE Volume LV Year 2015 Number 1 2015 Instytut Odlewnictwa. All rights reserved. DOI: 10.7356/iod.2015.01 Wpływ wskaźnika struktury WB
THERMODYNAMICS OF OXYGEN IN DILUTE LIQUID SILVER-TELLURIUM ALLOYS
 AGH University of Science and Technology Faculty of Non-Ferrous Metals Laboratory of Physical Chemistry and Electrochemistry THERMODYNAMICS OF OXYGEN IN DILUTE LIQUID SILVER-TELLURIUM ALLOYS Justyna Nyk,
AGH University of Science and Technology Faculty of Non-Ferrous Metals Laboratory of Physical Chemistry and Electrochemistry THERMODYNAMICS OF OXYGEN IN DILUTE LIQUID SILVER-TELLURIUM ALLOYS Justyna Nyk,
Tychy, plan miasta: Skala 1: (Polish Edition)
 Tychy, plan miasta: Skala 1:20 000 (Polish Edition) Poland) Przedsiebiorstwo Geodezyjno-Kartograficzne (Katowice Click here if your download doesn"t start automatically Tychy, plan miasta: Skala 1:20 000
Tychy, plan miasta: Skala 1:20 000 (Polish Edition) Poland) Przedsiebiorstwo Geodezyjno-Kartograficzne (Katowice Click here if your download doesn"t start automatically Tychy, plan miasta: Skala 1:20 000
Zakopane, plan miasta: Skala ok. 1: = City map (Polish Edition)
 Zakopane, plan miasta: Skala ok. 1:15 000 = City map (Polish Edition) Click here if your download doesn"t start automatically Zakopane, plan miasta: Skala ok. 1:15 000 = City map (Polish Edition) Zakopane,
Zakopane, plan miasta: Skala ok. 1:15 000 = City map (Polish Edition) Click here if your download doesn"t start automatically Zakopane, plan miasta: Skala ok. 1:15 000 = City map (Polish Edition) Zakopane,
Helena Boguta, klasa 8W, rok szkolny 2018/2019
 Poniższy zbiór zadań został wykonany w ramach projektu Mazowiecki program stypendialny dla uczniów szczególnie uzdolnionych - najlepsza inwestycja w człowieka w roku szkolnym 2018/2019. Składają się na
Poniższy zbiór zadań został wykonany w ramach projektu Mazowiecki program stypendialny dla uczniów szczególnie uzdolnionych - najlepsza inwestycja w człowieka w roku szkolnym 2018/2019. Składają się na
Microsystems in Medical Applications Liquid Flow Sensors
 Microsystems in Medical Applications Liquid Flow Sensors Prezentacja multimedialna współfinansowana przez Unię Europejską w ramach Europejskiego Funduszu Społecznego w projekcie pt. Innowacyjna dydaktyka
Microsystems in Medical Applications Liquid Flow Sensors Prezentacja multimedialna współfinansowana przez Unię Europejską w ramach Europejskiego Funduszu Społecznego w projekcie pt. Innowacyjna dydaktyka
 aforementioned device she also has to estimate the time when the patients need the infusion to be replaced and/or disconnected. Meanwhile, however, she must cope with many other tasks. If the department
aforementioned device she also has to estimate the time when the patients need the infusion to be replaced and/or disconnected. Meanwhile, however, she must cope with many other tasks. If the department
PRACE INSTYTUTU ODLEWNICTWA TRANSACTIONS OF FOUNDRY RESEARCH INSTITUTE
 PRACE INSTYTUTU ODLEWNICTWA TRANSACTIONS OF FOUNDRY RESEARCH INSTITUTE Volume LV Year 2015 Number 3 2015 Instytut Odlewnictwa. All rights reserved. DOI: 10.7356/iod.2015.13 Ocena zwilżalności kwarcu przez
PRACE INSTYTUTU ODLEWNICTWA TRANSACTIONS OF FOUNDRY RESEARCH INSTITUTE Volume LV Year 2015 Number 3 2015 Instytut Odlewnictwa. All rights reserved. DOI: 10.7356/iod.2015.13 Ocena zwilżalności kwarcu przez
TYRE PYROLYSIS. REDUXCO GENERAL DISTRIBUTOR :: ::
 TYRE PYROLYSIS Installation for rubber waste pyrolysis designed for processing of used tyres and plastic waste (polyethylene, polypropylene, polystyrene), where the final product could be electricity,
TYRE PYROLYSIS Installation for rubber waste pyrolysis designed for processing of used tyres and plastic waste (polyethylene, polypropylene, polystyrene), where the final product could be electricity,
The economical and ecological aspects of using the modified water-glass
 TEKA. COMMISSION OF MOTORIZATION AND ENERGETICS IN AGRICULTURE, Vol., No., 67 7 The economical and ecological aspects of using the modified water-glass Irena Izdebska-Szanda * * Foundry Research Institute,
TEKA. COMMISSION OF MOTORIZATION AND ENERGETICS IN AGRICULTURE, Vol., No., 67 7 The economical and ecological aspects of using the modified water-glass Irena Izdebska-Szanda * * Foundry Research Institute,
Cracow University of Economics Poland. Overview. Sources of Real GDP per Capita Growth: Polish Regional-Macroeconomic Dimensions 2000-2005
 Cracow University of Economics Sources of Real GDP per Capita Growth: Polish Regional-Macroeconomic Dimensions 2000-2005 - Key Note Speech - Presented by: Dr. David Clowes The Growth Research Unit CE Europe
Cracow University of Economics Sources of Real GDP per Capita Growth: Polish Regional-Macroeconomic Dimensions 2000-2005 - Key Note Speech - Presented by: Dr. David Clowes The Growth Research Unit CE Europe
Revenue Maximization. Sept. 25, 2018
 Revenue Maximization Sept. 25, 2018 Goal So Far: Ideal Auctions Dominant-Strategy Incentive Compatible (DSIC) b i = v i is a dominant strategy u i 0 x is welfare-maximizing x and p run in polynomial time
Revenue Maximization Sept. 25, 2018 Goal So Far: Ideal Auctions Dominant-Strategy Incentive Compatible (DSIC) b i = v i is a dominant strategy u i 0 x is welfare-maximizing x and p run in polynomial time
Technika jonowego rozpylenia
 Technika jonowego rozpylenia dr K.Marszałek 1 Technika jonowego rozpylenia dr K.Marszałek 2 Mechanizm rozpylenia dr K.Marszałek 3 :Création et transferet des différentes espèces en pulvérisation cathodique
Technika jonowego rozpylenia dr K.Marszałek 1 Technika jonowego rozpylenia dr K.Marszałek 2 Mechanizm rozpylenia dr K.Marszałek 3 :Création et transferet des différentes espèces en pulvérisation cathodique
Weronika Mysliwiec, klasa 8W, rok szkolny 2018/2019
 Poniższy zbiór zadań został wykonany w ramach projektu Mazowiecki program stypendialny dla uczniów szczególnie uzdolnionych - najlepsza inwestycja w człowieka w roku szkolnym 2018/2019. Tresci zadań rozwiązanych
Poniższy zbiór zadań został wykonany w ramach projektu Mazowiecki program stypendialny dla uczniów szczególnie uzdolnionych - najlepsza inwestycja w człowieka w roku szkolnym 2018/2019. Tresci zadań rozwiązanych
H γ0 - the standard enthalpy of g phase. H β γ - the transition enthalpy of b to g
 H α0 - the standard enthalpy of a phase H β0 - the standard enthalpy of b phase H γ0 - the standard enthalpy of g phase H α β - the transition enthalpy of a to b phase at T t1 H β γ - the transition enthalpy
H α0 - the standard enthalpy of a phase H β0 - the standard enthalpy of b phase H γ0 - the standard enthalpy of g phase H α β - the transition enthalpy of a to b phase at T t1 H β γ - the transition enthalpy
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume Issue 4
 A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 54 2009 Issue 4 D. LEŚNIAK STRUCTURE AND MECHANICAL PROPERTIES OF EXTRUDED AlCuMg SECTIONS IN T5 TEMPER STRUKTURA I WŁASNOŚCI MECHANICZNE
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 54 2009 Issue 4 D. LEŚNIAK STRUCTURE AND MECHANICAL PROPERTIES OF EXTRUDED AlCuMg SECTIONS IN T5 TEMPER STRUKTURA I WŁASNOŚCI MECHANICZNE
PROCEEDINGS OF THE INSTITUTE OF VEHICLES 2(106)/2016 (12 pt)
 PROCEEDINGS OF THE INSTITUTE OF VEHICLES 2(106)/2016 Maciej Foremny 1, Szymon Gudowski 2, Michał Malesza 3, Henryk Bąkowski 4 TRIBOLOGICAL WEAR ESTIMATION OF THE ENGINE OILS USED IN DRIFTING 1. Introduction
PROCEEDINGS OF THE INSTITUTE OF VEHICLES 2(106)/2016 Maciej Foremny 1, Szymon Gudowski 2, Michał Malesza 3, Henryk Bąkowski 4 TRIBOLOGICAL WEAR ESTIMATION OF THE ENGINE OILS USED IN DRIFTING 1. Introduction
WYKAZ DOROBKU NAUKOWEGO
 SUMMARY Straight majority of technologies of industrially important products is based on reactions of catalytic character, one of such processes is dehydrogenation. Of substantial importance is for example
SUMMARY Straight majority of technologies of industrially important products is based on reactions of catalytic character, one of such processes is dehydrogenation. Of substantial importance is for example
KRYTERIA OCENY WYBIJALNOŚCI MAS ZE SZKŁEM WODNYM
 18/17 ARCHIWUM ODLEWNICTWA Rok 2005, Rocznik 5, Nr 17 Archives of Foundry Year 2005, Volume 5, Book 17 PAN - Katowice PL ISSN 1642-5308 KRYTERIA OCENY WYBIJALNOŚCI MAS ZE SZKŁEM WODNYM K. MAJOR-GABRYŚ
18/17 ARCHIWUM ODLEWNICTWA Rok 2005, Rocznik 5, Nr 17 Archives of Foundry Year 2005, Volume 5, Book 17 PAN - Katowice PL ISSN 1642-5308 KRYTERIA OCENY WYBIJALNOŚCI MAS ZE SZKŁEM WODNYM K. MAJOR-GABRYŚ
Patients price acceptance SELECTED FINDINGS
 Patients price acceptance SELECTED FINDINGS October 2015 Summary With growing economy and Poles benefiting from this growth, perception of prices changes - this is also true for pharmaceuticals It may
Patients price acceptance SELECTED FINDINGS October 2015 Summary With growing economy and Poles benefiting from this growth, perception of prices changes - this is also true for pharmaceuticals It may
ERASMUS + : Trail of extinct and active volcanoes, earthquakes through Europe. SURVEY TO STUDENTS.
 ERASMUS + : Trail of extinct and active volcanoes, earthquakes through Europe. SURVEY TO STUDENTS. Strona 1 1. Please give one answer. I am: Students involved in project 69% 18 Student not involved in
ERASMUS + : Trail of extinct and active volcanoes, earthquakes through Europe. SURVEY TO STUDENTS. Strona 1 1. Please give one answer. I am: Students involved in project 69% 18 Student not involved in
TECHNICAL CATALOGUE WHITEHEART MALLEABLE CAST IRON FITTINGS EE
 TECHNICAL CATALOGUE WHITEHEART MALLEABLE CAST IRON FITTINGS EE Poland GENERAL INFORMATION USE Whiteheart malleable cast iron fittings brand EE are used in threaded pipe joints, particularly in water, gas,
TECHNICAL CATALOGUE WHITEHEART MALLEABLE CAST IRON FITTINGS EE Poland GENERAL INFORMATION USE Whiteheart malleable cast iron fittings brand EE are used in threaded pipe joints, particularly in water, gas,
Tytuł pracy w języku angielskim: Physical properties of liquid crystal mixtures of chiral and achiral compounds for use in LCDs
 Dr inż. Jan Czerwiec Kierownik pracy: dr hab. Monika Marzec Tytuł pracy w języku polskim: Właściwości fizyczne mieszanin ciekłokrystalicznych związków chiralnych i achiralnych w odniesieniu do zastosowań
Dr inż. Jan Czerwiec Kierownik pracy: dr hab. Monika Marzec Tytuł pracy w języku polskim: Właściwości fizyczne mieszanin ciekłokrystalicznych związków chiralnych i achiralnych w odniesieniu do zastosowań
Karpacz, plan miasta 1:10 000: Panorama Karkonoszy, mapa szlakow turystycznych (Polish Edition)
 Karpacz, plan miasta 1:10 000: Panorama Karkonoszy, mapa szlakow turystycznych (Polish Edition) J Krupski Click here if your download doesn"t start automatically Karpacz, plan miasta 1:10 000: Panorama
Karpacz, plan miasta 1:10 000: Panorama Karkonoszy, mapa szlakow turystycznych (Polish Edition) J Krupski Click here if your download doesn"t start automatically Karpacz, plan miasta 1:10 000: Panorama
WENTYLATORY PROMIENIOWE SINGLE-INLET DRUM BĘBNOWE JEDNOSTRUMIENIOWE CENTRIFUGAL FAN
 WENTYLATORY PROMIENIOWE SINGLE-INLET DRUM BĘBNOWE JEDNOSTRUMIENIOWE CENTRIFUGAL FAN TYP WPB TYPE WPB Wentylatory promieniowe jednostrumieniowe bębnowe (z wirnikiem typu Single-inlet centrifugal fans (with
WENTYLATORY PROMIENIOWE SINGLE-INLET DRUM BĘBNOWE JEDNOSTRUMIENIOWE CENTRIFUGAL FAN TYP WPB TYPE WPB Wentylatory promieniowe jednostrumieniowe bębnowe (z wirnikiem typu Single-inlet centrifugal fans (with
Volume Issue 2 THE EFFECT OF DYNAMIC WETTING IN A SAND BINDER SYSTEM ON THE SAND MOULD STRENGTH
 A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 54 2009 Issue 2 B. HUTERA THE EFFECT OF DYNAMIC WETTING IN A SAND BINDER SYSTEM ON THE SAND MOULD STRENGTH WPŁYW DYNAMIKI ZWILŻANIA
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 54 2009 Issue 2 B. HUTERA THE EFFECT OF DYNAMIC WETTING IN A SAND BINDER SYSTEM ON THE SAND MOULD STRENGTH WPŁYW DYNAMIKI ZWILŻANIA
Proposal of thesis topic for mgr in. (MSE) programme in Telecommunications and Computer Science
 Proposal of thesis topic for mgr in (MSE) programme 1 Topic: Monte Carlo Method used for a prognosis of a selected technological process 2 Supervisor: Dr in Małgorzata Langer 3 Auxiliary supervisor: 4
Proposal of thesis topic for mgr in (MSE) programme 1 Topic: Monte Carlo Method used for a prognosis of a selected technological process 2 Supervisor: Dr in Małgorzata Langer 3 Auxiliary supervisor: 4
Knovel Math: Jakość produktu
 Knovel Math: Jakość produktu Knovel jest agregatorem materiałów pełnotekstowych dostępnych w formacie PDF i interaktywnym. Narzędzia interaktywne Knovel nie są stworzone wokół specjalnych algorytmów wymagających
Knovel Math: Jakość produktu Knovel jest agregatorem materiałów pełnotekstowych dostępnych w formacie PDF i interaktywnym. Narzędzia interaktywne Knovel nie są stworzone wokół specjalnych algorytmów wymagających
Machine Learning for Data Science (CS4786) Lecture 11. Spectral Embedding + Clustering
 Machine Learning for Data Science (CS4786) Lecture 11 Spectral Embedding + Clustering MOTIVATING EXAMPLE What can you say from this network? MOTIVATING EXAMPLE How about now? THOUGHT EXPERIMENT For each
Machine Learning for Data Science (CS4786) Lecture 11 Spectral Embedding + Clustering MOTIVATING EXAMPLE What can you say from this network? MOTIVATING EXAMPLE How about now? THOUGHT EXPERIMENT For each
Rozpoznawanie twarzy metodą PCA Michał Bereta 1. Testowanie statystycznej istotności różnic między jakością klasyfikatorów
 Rozpoznawanie twarzy metodą PCA Michał Bereta www.michalbereta.pl 1. Testowanie statystycznej istotności różnic między jakością klasyfikatorów Wiemy, że możemy porównywad klasyfikatory np. za pomocą kroswalidacji.
Rozpoznawanie twarzy metodą PCA Michał Bereta www.michalbereta.pl 1. Testowanie statystycznej istotności różnic między jakością klasyfikatorów Wiemy, że możemy porównywad klasyfikatory np. za pomocą kroswalidacji.
 www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
Medical electronics part 10 Physiological transducers
 Medical electronics part 10 Prezentacja multimedialna współfinansowana przez Unię Europejską w ramach Europejskiego Funduszu Społecznego w projekcie pt. Innowacyjna dydaktyka bez ograniczeń - zintegrowany
Medical electronics part 10 Prezentacja multimedialna współfinansowana przez Unię Europejską w ramach Europejskiego Funduszu Społecznego w projekcie pt. Innowacyjna dydaktyka bez ograniczeń - zintegrowany
SSW1.1, HFW Fry #20, Zeno #25 Benchmark: Qtr.1. Fry #65, Zeno #67. like
 SSW1.1, HFW Fry #20, Zeno #25 Benchmark: Qtr.1 I SSW1.1, HFW Fry #65, Zeno #67 Benchmark: Qtr.1 like SSW1.2, HFW Fry #47, Zeno #59 Benchmark: Qtr.1 do SSW1.2, HFW Fry #5, Zeno #4 Benchmark: Qtr.1 to SSW1.2,
SSW1.1, HFW Fry #20, Zeno #25 Benchmark: Qtr.1 I SSW1.1, HFW Fry #65, Zeno #67 Benchmark: Qtr.1 like SSW1.2, HFW Fry #47, Zeno #59 Benchmark: Qtr.1 do SSW1.2, HFW Fry #5, Zeno #4 Benchmark: Qtr.1 to SSW1.2,
Fig 5 Spectrograms of the original signal (top) extracted shaft-related GAD components (middle) and
 Fig 4 Measured vibration signal (top). Blue original signal. Red component related to periodic excitation of resonances and noise. Green component related. Rotational speed profile used for experiment
Fig 4 Measured vibration signal (top). Blue original signal. Red component related to periodic excitation of resonances and noise. Green component related. Rotational speed profile used for experiment
Wojewodztwo Koszalinskie: Obiekty i walory krajoznawcze (Inwentaryzacja krajoznawcza Polski) (Polish Edition)
 Wojewodztwo Koszalinskie: Obiekty i walory krajoznawcze (Inwentaryzacja krajoznawcza Polski) (Polish Edition) Robert Respondowski Click here if your download doesn"t start automatically Wojewodztwo Koszalinskie:
Wojewodztwo Koszalinskie: Obiekty i walory krajoznawcze (Inwentaryzacja krajoznawcza Polski) (Polish Edition) Robert Respondowski Click here if your download doesn"t start automatically Wojewodztwo Koszalinskie:
Hard-Margin Support Vector Machines
 Hard-Margin Support Vector Machines aaacaxicbzdlssnafiyn9vbjlepk3ay2gicupasvu4iblxuaw2hjmuwn7ddjjmxm1bkcg1/fjqsvt76fo9/gazqfvn8y+pjpozw5vx8zkpvtfxmlhcwl5zxyqrm2vrg5zw3vxmsoezi4ogkr6phieky5crvvjhriqvdom9l2xxftevuwcekj3lktmhghgniauiyutvrwxtvme34a77kbvg73gtygpjsrfati1+xc8c84bvraowbf+uwnipyehcvmkjrdx46vlykhkgykm3ujjdhcyzqkxy0chur6ax5cbg+1m4bbjptjcubuz4kuhvjoql93hkin5hxtav5x6yyqopnsyuneey5ni4keqrxbar5wqaxbik00icyo/iveiyqqvjo1u4fgzj/8f9x67bzmxnurjzmijtlybwfgcdjgfdtajwgcf2dwaj7ac3g1ho1n4814n7wwjgjmf/ys8fenfycuzq==
Hard-Margin Support Vector Machines aaacaxicbzdlssnafiyn9vbjlepk3ay2gicupasvu4iblxuaw2hjmuwn7ddjjmxm1bkcg1/fjqsvt76fo9/gazqfvn8y+pjpozw5vx8zkpvtfxmlhcwl5zxyqrm2vrg5zw3vxmsoezi4ogkr6phieky5crvvjhriqvdom9l2xxftevuwcekj3lktmhghgniauiyutvrwxtvme34a77kbvg73gtygpjsrfati1+xc8c84bvraowbf+uwnipyehcvmkjrdx46vlykhkgykm3ujjdhcyzqkxy0chur6ax5cbg+1m4bbjptjcubuz4kuhvjoql93hkin5hxtav5x6yyqopnsyuneey5ni4keqrxbar5wqaxbik00icyo/iveiyqqvjo1u4fgzj/8f9x67bzmxnurjzmijtlybwfgcdjgfdtajwgcf2dwaj7ac3g1ho1n4814n7wwjgjmf/ys8fenfycuzq==
Zarządzanie sieciami telekomunikacyjnymi
 SNMP Protocol The Simple Network Management Protocol (SNMP) is an application layer protocol that facilitates the exchange of management information between network devices. It is part of the Transmission
SNMP Protocol The Simple Network Management Protocol (SNMP) is an application layer protocol that facilitates the exchange of management information between network devices. It is part of the Transmission
Sargent Opens Sonairte Farmers' Market
 Sargent Opens Sonairte Farmers' Market 31 March, 2008 1V8VIZSV7EVKIRX8(1MRMWXIVSJ7XEXIEXXLI(ITEVXQIRXSJ%KVMGYPXYVI *MWLIVMIWERH*SSHTIVJSVQIHXLISJJMGMEPSTIRMRKSJXLI7SREMVXI*EVQIVW 1EVOIXMR0E]XS[R'S1IEXL
Sargent Opens Sonairte Farmers' Market 31 March, 2008 1V8VIZSV7EVKIRX8(1MRMWXIVSJ7XEXIEXXLI(ITEVXQIRXSJ%KVMGYPXYVI *MWLIVMIWERH*SSHTIVJSVQIHXLISJJMGMEPSTIRMRKSJXLI7SREMVXI*EVQIVW 1EVOIXMR0E]XS[R'S1IEXL
OpenPoland.net API Documentation
 OpenPoland.net API Documentation Release 1.0 Michał Gryczka July 11, 2014 Contents 1 REST API tokens: 3 1.1 How to get a token............................................ 3 2 REST API : search for assets
OpenPoland.net API Documentation Release 1.0 Michał Gryczka July 11, 2014 Contents 1 REST API tokens: 3 1.1 How to get a token............................................ 3 2 REST API : search for assets
Krytyczne czynniki sukcesu w zarządzaniu projektami
 Seweryn SPAŁEK Krytyczne czynniki sukcesu w zarządzaniu projektami MONOGRAFIA Wydawnictwo Politechniki Śląskiej Gliwice 2004 SPIS TREŚCI WPROWADZENIE 5 1. ZARZĄDZANIE PROJEKTAMI W ORGANIZACJI 13 1.1. Zarządzanie
Seweryn SPAŁEK Krytyczne czynniki sukcesu w zarządzaniu projektami MONOGRAFIA Wydawnictwo Politechniki Śląskiej Gliwice 2004 SPIS TREŚCI WPROWADZENIE 5 1. ZARZĄDZANIE PROJEKTAMI W ORGANIZACJI 13 1.1. Zarządzanie
ROZPRAWY NR 128. Stanis³aw Mroziñski
 UNIWERSYTET TECHNOLOGICZNO-PRZYRODNICZY IM. JANA I JÊDRZEJA ŒNIADECKICH W BYDGOSZCZY ROZPRAWY NR 128 Stanis³aw Mroziñski STABILIZACJA W ASNOŒCI CYKLICZNYCH METALI I JEJ WP YW NA TRWA OŒÆ ZMÊCZENIOW BYDGOSZCZ
UNIWERSYTET TECHNOLOGICZNO-PRZYRODNICZY IM. JANA I JÊDRZEJA ŒNIADECKICH W BYDGOSZCZY ROZPRAWY NR 128 Stanis³aw Mroziñski STABILIZACJA W ASNOŒCI CYKLICZNYCH METALI I JEJ WP YW NA TRWA OŒÆ ZMÊCZENIOW BYDGOSZCZ
 www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
PROPERTIES OF PARTICLEBOARDS RESINATED WITH PMDI MODIFIED WITH POLYETHYLENE GLYCOLS
 SCIENTIARUM POLONORUMACTA Silv. Colendar. Rat. Ind. Lignar. 6(4) 27, 11-16 PROPERTIES OF PARTICLEBOARDS RESINATED WITH MODIFIED WITH POLYETHYLENE GLYCOLS Dorota Dziurka 1, Marek Jabłoński 2, Radosław Mirski
SCIENTIARUM POLONORUMACTA Silv. Colendar. Rat. Ind. Lignar. 6(4) 27, 11-16 PROPERTIES OF PARTICLEBOARDS RESINATED WITH MODIFIED WITH POLYETHYLENE GLYCOLS Dorota Dziurka 1, Marek Jabłoński 2, Radosław Mirski
Towards Stability Analysis of Data Transport Mechanisms: a Fluid Model and an Application
 Towards Stability Analysis of Data Transport Mechanisms: a Fluid Model and an Application Gayane Vardoyan *, C. V. Hollot, Don Towsley* * College of Information and Computer Sciences, Department of Electrical
Towards Stability Analysis of Data Transport Mechanisms: a Fluid Model and an Application Gayane Vardoyan *, C. V. Hollot, Don Towsley* * College of Information and Computer Sciences, Department of Electrical
Wydział Fizyki, Astronomii i Informatyki Stosowanej Uniwersytet Mikołaja Kopernika w Toruniu
 IONS-14 / OPTO Meeting For Young Researchers 2013 Khet Tournament On 3-6 July 2013 at the Faculty of Physics, Astronomy and Informatics of Nicolaus Copernicus University in Torun (Poland) there were two
IONS-14 / OPTO Meeting For Young Researchers 2013 Khet Tournament On 3-6 July 2013 at the Faculty of Physics, Astronomy and Informatics of Nicolaus Copernicus University in Torun (Poland) there were two
DETECTION OF MATERIAL INTEGRATED CONDUCTORS FOR CONNECTIVE RIVETING OF FUNCTION-INTEGRATIVE TEXTILE-REINFORCED THERMOPLASTIC COMPOSITES
 Kompozyty 11: 2 (2011) 152-156 Werner A. Hufenbach, Frank Adam, Maik Gude, Ivonne Körner, Thomas Heber*, Anja Winkler Technische Universität Dresden, Institute of Lightweight Engineering and Polymer Technology
Kompozyty 11: 2 (2011) 152-156 Werner A. Hufenbach, Frank Adam, Maik Gude, Ivonne Körner, Thomas Heber*, Anja Winkler Technische Universität Dresden, Institute of Lightweight Engineering and Polymer Technology
Ocena potrzeb pacjentów z zaburzeniami psychicznymi
 Mikołaj Trizna Ocena potrzeb pacjentów z zaburzeniami psychicznymi przebywających na oddziałach psychiatrii sądowej Rozprawa na stopień doktora nauk medycznych Promotor: dr hab.n.med. Tomasz Adamowski,
Mikołaj Trizna Ocena potrzeb pacjentów z zaburzeniami psychicznymi przebywających na oddziałach psychiatrii sądowej Rozprawa na stopień doktora nauk medycznych Promotor: dr hab.n.med. Tomasz Adamowski,
WPŁYW WARUNKÓW UTWARDZANIA I GRUBOŚCI UTWARDZONEJ WARSTEWKI NA WYTRZYMAŁOŚĆ NA ROZCIĄGANIE ŻYWICY SYNTETYCZNEJ
 61/2 Archives of Foundry, Year 21, Volume 1, 1 (2/2) Archiwum Odlewnictwa, Rok 21, Rocznik 1, Nr 1 (2/2) PAN Katowice PL ISSN 1642-58 WPŁYW WARUNKÓW UTWARDZANIA I GRUBOŚCI UTWARDZONEJ WARSTEWKI NA WYTRZYMAŁOŚĆ
61/2 Archives of Foundry, Year 21, Volume 1, 1 (2/2) Archiwum Odlewnictwa, Rok 21, Rocznik 1, Nr 1 (2/2) PAN Katowice PL ISSN 1642-58 WPŁYW WARUNKÓW UTWARDZANIA I GRUBOŚCI UTWARDZONEJ WARSTEWKI NA WYTRZYMAŁOŚĆ
INSPECTION METHODS FOR QUALITY CONTROL OF FIBRE METAL LAMINATES IN AEROSPACE COMPONENTS
 Kompozyty 11: 2 (2011) 130-135 Krzysztof Dragan 1 * Jarosław Bieniaś 2, Michał Sałaciński 1, Piotr Synaszko 1 1 Air Force Institute of Technology, Non Destructive Testing Lab., ul. ks. Bolesława 6, 01-494
Kompozyty 11: 2 (2011) 130-135 Krzysztof Dragan 1 * Jarosław Bieniaś 2, Michał Sałaciński 1, Piotr Synaszko 1 1 Air Force Institute of Technology, Non Destructive Testing Lab., ul. ks. Bolesława 6, 01-494
Stargard Szczecinski i okolice (Polish Edition)
 Stargard Szczecinski i okolice (Polish Edition) Janusz Leszek Jurkiewicz Click here if your download doesn"t start automatically Stargard Szczecinski i okolice (Polish Edition) Janusz Leszek Jurkiewicz
Stargard Szczecinski i okolice (Polish Edition) Janusz Leszek Jurkiewicz Click here if your download doesn"t start automatically Stargard Szczecinski i okolice (Polish Edition) Janusz Leszek Jurkiewicz
yft Lublin, June 1999
 Waldemar STAROŃ Janusz MIELNIK Henryk GURGUL Department of Sea Physics Chair of Physics Szczecin University Wielkopolska 15,70-451 Szczecin POLAND POLISH POLAR STUDIES XXVI Polar Symposium yft Lublin,
Waldemar STAROŃ Janusz MIELNIK Henryk GURGUL Department of Sea Physics Chair of Physics Szczecin University Wielkopolska 15,70-451 Szczecin POLAND POLISH POLAR STUDIES XXVI Polar Symposium yft Lublin,
QUANTITATIVE AND QUALITATIVE CHARACTERISTICS OF FINGERPRINT BIOMETRIC TEMPLATES
 ZESZYTY NAUKOWE POLITECHNIKI ŚLĄSKIEJ 2014 Seria: ORGANIZACJA I ZARZĄDZANIE z. 74 Nr kol. 1921 Adrian KAPCZYŃSKI Politechnika Śląska Instytut Ekonomii i Informatyki QUANTITATIVE AND QUALITATIVE CHARACTERISTICS
ZESZYTY NAUKOWE POLITECHNIKI ŚLĄSKIEJ 2014 Seria: ORGANIZACJA I ZARZĄDZANIE z. 74 Nr kol. 1921 Adrian KAPCZYŃSKI Politechnika Śląska Instytut Ekonomii i Informatyki QUANTITATIVE AND QUALITATIVE CHARACTERISTICS
DM-ML, DM-FL. Auxiliary Equipment and Accessories. Damper Drives. Dimensions. Descritpion
 DM-ML, DM-FL Descritpion DM-ML and DM-FL actuators are designed for driving round dampers and square multi-blade dampers. Example identification Product code: DM-FL-5-2 voltage Dimensions DM-ML-6 DM-ML-8
DM-ML, DM-FL Descritpion DM-ML and DM-FL actuators are designed for driving round dampers and square multi-blade dampers. Example identification Product code: DM-FL-5-2 voltage Dimensions DM-ML-6 DM-ML-8
Relaxation of the Cosmological Constant
 Relaxation of the Cosmological Constant with Peter Graham and David E. Kaplan The Born Again Universe + Work in preparation + Work in progress aaab7nicdvbns8nafhypx7v+vt16wsycp5kioseifw8ekthwaepzbf7apztn2n0ipfrhepggifd/jzf/jzs2brudwbhm5rhvtzakro3rfjqlpewv1bxyemvjc2t7p7q719zjphi2wcisdr9qjyjlbblubn6ncmkccoweo6vc7zyg0jyrd2acoh/tgeqrz9ryqdo7sdgq9qs1t37m5ibu3v2qqvekpqyfmv3qry9mwbajnexqrbuemxp/qpxhtoc00ss0ppsn6ac7lkoao/yns3wn5mgqiykszz80zkz+n5jqwotxhnhktm1q//zy8s+vm5nowp9wmwygjzt/fgwcmitkt5oqk2rgjc2hthg7k2fdqigztqgklwfxkfmfte/qnuw3p7xgzvfhgq7gei7bg3nowdu0oqumrvaiz/dipm6t8+q8zamlp5jzhx9w3r8agjmpzw==
Relaxation of the Cosmological Constant with Peter Graham and David E. Kaplan The Born Again Universe + Work in preparation + Work in progress aaab7nicdvbns8nafhypx7v+vt16wsycp5kioseifw8ekthwaepzbf7apztn2n0ipfrhepggifd/jzf/jzs2brudwbhm5rhvtzakro3rfjqlpewv1bxyemvjc2t7p7q719zjphi2wcisdr9qjyjlbblubn6ncmkccoweo6vc7zyg0jyrd2acoh/tgeqrz9ryqdo7sdgq9qs1t37m5ibu3v2qqvekpqyfmv3qry9mwbajnexqrbuemxp/qpxhtoc00ss0ppsn6ac7lkoao/yns3wn5mgqiykszz80zkz+n5jqwotxhnhktm1q//zy8s+vm5nowp9wmwygjzt/fgwcmitkt5oqk2rgjc2hthg7k2fdqigztqgklwfxkfmfte/qnuw3p7xgzvfhgq7gei7bg3nowdu0oqumrvaiz/dipm6t8+q8zamlp5jzhx9w3r8agjmpzw==
MaPlan Sp. z O.O. Click here if your download doesn"t start automatically
 Mierzeja Wislana, mapa turystyczna 1:50 000: Mikoszewo, Jantar, Stegna, Sztutowo, Katy Rybackie, Przebrno, Krynica Morska, Piaski, Frombork =... = Carte touristique (Polish Edition) MaPlan Sp. z O.O Click
Mierzeja Wislana, mapa turystyczna 1:50 000: Mikoszewo, Jantar, Stegna, Sztutowo, Katy Rybackie, Przebrno, Krynica Morska, Piaski, Frombork =... = Carte touristique (Polish Edition) MaPlan Sp. z O.O Click
Network Services for Spatial Data in European Geo-Portals and their Compliance with ISO and OGC Standards
 INSPIRE Conference 2010 INSPIRE as a Framework for Cooperation Network Services for Spatial Data in European Geo-Portals and their Compliance with ISO and OGC Standards Elżbieta Bielecka Agnieszka Zwirowicz
INSPIRE Conference 2010 INSPIRE as a Framework for Cooperation Network Services for Spatial Data in European Geo-Portals and their Compliance with ISO and OGC Standards Elżbieta Bielecka Agnieszka Zwirowicz
II wariant dwie skale ocen II alternative two grading scales
 Kryteria przeliczania uzyskanych przez kandydata ocen na punkty do listy rankingowej University Criteria for converting candidates grades into the points for the Ranking List Wymagane przedmioty : fizyka,
Kryteria przeliczania uzyskanych przez kandydata ocen na punkty do listy rankingowej University Criteria for converting candidates grades into the points for the Ranking List Wymagane przedmioty : fizyka,
Few-fermion thermometry
 Few-fermion thermometry Phys. Rev. A 97, 063619 (2018) Tomasz Sowiński Institute of Physics of the Polish Academy of Sciences Co-authors: Marcin Płodzień Rafał Demkowicz-Dobrzański FEW-BODY PROBLEMS FewBody.ifpan.edu.pl
Few-fermion thermometry Phys. Rev. A 97, 063619 (2018) Tomasz Sowiński Institute of Physics of the Polish Academy of Sciences Co-authors: Marcin Płodzień Rafał Demkowicz-Dobrzański FEW-BODY PROBLEMS FewBody.ifpan.edu.pl
CERAMIC POROUS MATERIALS MADE OF ZnO INTENDED FOR ELIMINATING PARTICLES IMITATING VIRUSES FROM WATER
 13: 1 (2013) 72-76 Milena Zalewska*, Patrycja Kamińska, Mikołaj Szafran Warsaw University of Technology, Faculty of Chemistry, Department of Inorganic Technology and Ceramics ul. Noakowskiego 3, 00-664
13: 1 (2013) 72-76 Milena Zalewska*, Patrycja Kamińska, Mikołaj Szafran Warsaw University of Technology, Faculty of Chemistry, Department of Inorganic Technology and Ceramics ul. Noakowskiego 3, 00-664
Has the heat wave frequency or intensity changed in Poland since 1950?
 Has the heat wave frequency or intensity changed in Poland since 1950? Joanna Wibig Department of Meteorology and Climatology, University of Lodz, Poland OUTLINE: Motivation Data Heat wave frequency measures
Has the heat wave frequency or intensity changed in Poland since 1950? Joanna Wibig Department of Meteorology and Climatology, University of Lodz, Poland OUTLINE: Motivation Data Heat wave frequency measures
Analysis of Movie Profitability STAT 469 IN CLASS ANALYSIS #2
 Analysis of Movie Profitability STAT 469 IN CLASS ANALYSIS #2 aaaklnictzzjb9tgfmcnadpg7oy0lxa9edva9kkapdarhyk2k7gourinlwsweyzikuyiigvyleiv/cv767fpf/5crc1xt9va5mx7w3m/ecuqw1kuztpx/rl3/70h73/w4cog9dhhn3z62d6jzy+yzj766txpoir9nzszisjynetqr+rvlfvyoozu5xbybpsxb1wahul8phczdt2v4zgchb7uecwphlyigrgkjcyiflfyci0kxnmr4z6kw0jsokvot8isntpa3gbknlcufiv/h+hh+eur4fomd417rvtfjoit5pfju6yxiab2fmwk0y/feuybobqk+axnke8xzjjhfyd8kkpl9zdoddkazd5j6bzpemjb64smjb6vb4xmehysu08lsrszopxftlzee130jcb0zjxy7r5wa2f1s2off2+dyatrughnrtpkuprlcpu55zlxpss/yqe2eamjkcf0jye8w8yas0paf6t0t2i9stmcua+inbi2rt01tz22tubbqwidypvgz6piynkpobirkxgu54ibzoti4pkw2i5ow9lnuaoabhuxfxqhvnrj6w15tb3furnbm+scyxobjhr5pmj5j/w5ix9wsa2tlwx9alpshlunzjgnrwvqbpwzjl9wes+ptyn+ypy/jgskavtl8j0hz1djdhzwtpjbbvpr1zj7jpg6ve7zxfngj75zee0vmp9qm2uvgu/9zdofq6r+g8l4xctvo+v+xdrfr8oxiwutycu0qgyf8icuyvp/sixfi9zxe11vp6mrjjovpmxm6acrtbia+wjr9bevlgjwlz5xd3rfna9g06qytaoofk8olxbxc7xby2evqjmmk6pjvvzxmpbnct6+036xp5vdbrnbdqph8brlfn/n/khnfumhf6z1v7h/80yieukkd5j0un82t9mynxzmk0s/bzn4tacdziszdhwrl8x5ako8qp1n1zn0k6w2em0km9zj1i4yt1pt3xiprw85jmc2m1ut2geum6y6es2fwx6c+wlrpykblopbuj5nnr2byygfy5opllv4+jmm7s6u+tvhywbnb0kv2lt5th4xipmiij+y1toiyo7bo0d+vzvovjkp6aoejsubhj3qrp3fjd/m23pay8h218ibvx3nicofvd1xi86+kh6nb/b+hgsjp5+qwpurzlir15np66vmdehh6tyazdm1k/5ejtuvurgcqux6yc+qw/sbsaj7lkt4x9qmtp7euk6zbdedyuzu6ptsu2eeu3rxcz06uf6g8wyuveznhkbzynajbb7r7cbmla+jbtrst0ow2v6ntkwv8svnwqnu5pa3oxfeexf93739p93chq/fv+jr8r0d9brhpcxr2w88bvqbr41j6wvrb+u5dzjpvx+veoaxwptzp/8cen+xbg==
Analysis of Movie Profitability STAT 469 IN CLASS ANALYSIS #2 aaaklnictzzjb9tgfmcnadpg7oy0lxa9edva9kkapdarhyk2k7gourinlwsweyzikuyiigvyleiv/cv767fpf/5crc1xt9va5mx7w3m/ecuqw1kuztpx/rl3/70h73/w4cog9dhhn3z62d6jzy+yzj766txpoir9nzszisjynetqr+rvlfvyoozu5xbybpsxb1wahul8phczdt2v4zgchb7uecwphlyigrgkjcyiflfyci0kxnmr4z6kw0jsokvot8isntpa3gbknlcufiv/h+hh+eur4fomd417rvtfjoit5pfju6yxiab2fmwk0y/feuybobqk+axnke8xzjjhfyd8kkpl9zdoddkazd5j6bzpemjb64smjb6vb4xmehysu08lsrszopxftlzee130jcb0zjxy7r5wa2f1s2off2+dyatrughnrtpkuprlcpu55zlxpss/yqe2eamjkcf0jye8w8yas0paf6t0t2i9stmcua+inbi2rt01tz22tubbqwidypvgz6piynkpobirkxgu54ibzoti4pkw2i5ow9lnuaoabhuxfxqhvnrj6w15tb3furnbm+scyxobjhr5pmj5j/w5ix9wsa2tlwx9alpshlunzjgnrwvqbpwzjl9wes+ptyn+ypy/jgskavtl8j0hz1djdhzwtpjbbvpr1zj7jpg6ve7zxfngj75zee0vmp9qm2uvgu/9zdofq6r+g8l4xctvo+v+xdrfr8oxiwutycu0qgyf8icuyvp/sixfi9zxe11vp6mrjjovpmxm6acrtbia+wjr9bevlgjwlz5xd3rfna9g06qytaoofk8olxbxc7xby2evqjmmk6pjvvzxmpbnct6+036xp5vdbrnbdqph8brlfn/n/khnfumhf6z1v7h/80yieukkd5j0un82t9mynxzmk0s/bzn4tacdziszdhwrl8x5ako8qp1n1zn0k6w2em0km9zj1i4yt1pt3xiprw85jmc2m1ut2geum6y6es2fwx6c+wlrpykblopbuj5nnr2byygfy5opllv4+jmm7s6u+tvhywbnb0kv2lt5th4xipmiij+y1toiyo7bo0d+vzvovjkp6aoejsubhj3qrp3fjd/m23pay8h218ibvx3nicofvd1xi86+kh6nb/b+hgsjp5+qwpurzlir15np66vmdehh6tyazdm1k/5ejtuvurgcqux6yc+qw/sbsaj7lkt4x9qmtp7euk6zbdedyuzu6ptsu2eeu3rxcz06uf6g8wyuveznhkbzynajbb7r7cbmla+jbtrst0ow2v6ntkwv8svnwqnu5pa3oxfeexf93739p93chq/fv+jr8r0d9brhpcxr2w88bvqbr41j6wvrb+u5dzjpvx+veoaxwptzp/8cen+xbg==
Convolution semigroups with linear Jacobi parameters
 Convolution semigroups with linear Jacobi parameters Michael Anshelevich; Wojciech Młotkowski Texas A&M University; University of Wrocław February 14, 2011 Jacobi parameters. µ = measure with finite moments,
Convolution semigroups with linear Jacobi parameters Michael Anshelevich; Wojciech Młotkowski Texas A&M University; University of Wrocław February 14, 2011 Jacobi parameters. µ = measure with finite moments,
Akademia Morska w Szczecinie. Wydział Mechaniczny
 Akademia Morska w Szczecinie Wydział Mechaniczny ROZPRAWA DOKTORSKA mgr inż. Marcin Kołodziejski Analiza metody obsługiwania zarządzanego niezawodnością pędników azymutalnych platformy pływającej Promotor:
Akademia Morska w Szczecinie Wydział Mechaniczny ROZPRAWA DOKTORSKA mgr inż. Marcin Kołodziejski Analiza metody obsługiwania zarządzanego niezawodnością pędników azymutalnych platformy pływającej Promotor:
Domy inaczej pomyślane A different type of housing CEZARY SANKOWSKI
 Domy inaczej pomyślane A different type of housing CEZARY SANKOWSKI O tym, dlaczego warto budować pasywnie, komu budownictwo pasywne się opłaca, a kto się go boi, z architektem, Cezarym Sankowskim, rozmawia
Domy inaczej pomyślane A different type of housing CEZARY SANKOWSKI O tym, dlaczego warto budować pasywnie, komu budownictwo pasywne się opłaca, a kto się go boi, z architektem, Cezarym Sankowskim, rozmawia
Institutional Determinants of IncomeLevel Convergence in the European. Union: Are Institutions Responsible for Divergence Tendencies of Some
 Institutional Determinants of IncomeLevel Convergence in the European Union: Are Institutions Responsible for Divergence Tendencies of Some Countries? Dr Mariusz Próchniak Katedra Ekonomii II, Szkoła Główna
Institutional Determinants of IncomeLevel Convergence in the European Union: Are Institutions Responsible for Divergence Tendencies of Some Countries? Dr Mariusz Próchniak Katedra Ekonomii II, Szkoła Główna
WPŁYW MIESZANKI EGZOTERMICZNEJ NA BAZIE Na 2 B 4 O 7 I NaNO 3 NA WYTRZYMAŁOŚĆ NA ROZCIĄGANIE STOPU AlSi7Mg
 4/ Archives of Foundry, Year 006, Volume 6, Archiwum Odlewnictwa, Rok 006, Rocznik 6, Nr PAN Katowice PL ISSN 64-508 WPŁYW MIESZANKI EGZOTERMICZNEJ NA BAZIE Na B 4 O 7 I NaNO NA WYTRZYMAŁOŚĆ NA ROZCIĄGANIE
4/ Archives of Foundry, Year 006, Volume 6, Archiwum Odlewnictwa, Rok 006, Rocznik 6, Nr PAN Katowice PL ISSN 64-508 WPŁYW MIESZANKI EGZOTERMICZNEJ NA BAZIE Na B 4 O 7 I NaNO NA WYTRZYMAŁOŚĆ NA ROZCIĄGANIE
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume Issue 1 DOI: /v y
 A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 57 2012 Issue 1 DOI: 10.2478/v10172-011-0149-y G. BOCZKAL, P. MARECKI, M. PEREK-NOWAK THE INFLUENCE OF SOLDERING CONDITIONS ON CONDUCTIVITY,
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 57 2012 Issue 1 DOI: 10.2478/v10172-011-0149-y G. BOCZKAL, P. MARECKI, M. PEREK-NOWAK THE INFLUENCE OF SOLDERING CONDITIONS ON CONDUCTIVITY,
Tytuł pracy w języku angielskim: Microstructural characterization of Ag/X/Ag (X = Sn, In) joints obtained as the effect of diffusion soledering.
 Dr inż. Przemysław Skrzyniarz Kierownik pracy: Prof. dr hab. inż. Paweł Zięba Tytuł pracy w języku polskim: Charakterystyka mikrostruktury spoin Ag/X/Ag (X = Sn, In) uzyskanych w wyniku niskotemperaturowego
Dr inż. Przemysław Skrzyniarz Kierownik pracy: Prof. dr hab. inż. Paweł Zięba Tytuł pracy w języku polskim: Charakterystyka mikrostruktury spoin Ag/X/Ag (X = Sn, In) uzyskanych w wyniku niskotemperaturowego
Latent Dirichlet Allocation Models and their Evaluation IT for Practice 2016
 Latent Dirichlet Allocation Models and their Evaluation IT for Practice 2016 Paweł Lula Cracow University of Economics, Poland pawel.lula@uek.krakow.pl Latent Dirichlet Allocation (LDA) Documents Latent
Latent Dirichlet Allocation Models and their Evaluation IT for Practice 2016 Paweł Lula Cracow University of Economics, Poland pawel.lula@uek.krakow.pl Latent Dirichlet Allocation (LDA) Documents Latent
Wybrzeze Baltyku, mapa turystyczna 1: (Polish Edition)
 Wybrzeze Baltyku, mapa turystyczna 1:50 000 (Polish Edition) Click here if your download doesn"t start automatically Wybrzeze Baltyku, mapa turystyczna 1:50 000 (Polish Edition) Wybrzeze Baltyku, mapa
Wybrzeze Baltyku, mapa turystyczna 1:50 000 (Polish Edition) Click here if your download doesn"t start automatically Wybrzeze Baltyku, mapa turystyczna 1:50 000 (Polish Edition) Wybrzeze Baltyku, mapa
Installation of EuroCert software for qualified electronic signature
 Installation of EuroCert software for qualified electronic signature for Microsoft Windows systems Warsaw 28.08.2019 Content 1. Downloading and running the software for the e-signature... 3 a) Installer
Installation of EuroCert software for qualified electronic signature for Microsoft Windows systems Warsaw 28.08.2019 Content 1. Downloading and running the software for the e-signature... 3 a) Installer
PRZEWODNIK PO PRZEDMIOCIE. Negotiation techniques. Management. Stationary. II degree
 Politechnika Częstochowska, Wydział Zarządzania PRZEWODNIK PO PRZEDMIOCIE Nazwa przedmiotu Kierunek Forma studiów Poziom kwalifikacji Rok Semestr Jednostka prowadząca Osoba sporządzająca Profil Rodzaj
Politechnika Częstochowska, Wydział Zarządzania PRZEWODNIK PO PRZEDMIOCIE Nazwa przedmiotu Kierunek Forma studiów Poziom kwalifikacji Rok Semestr Jednostka prowadząca Osoba sporządzająca Profil Rodzaj
THEORETICAL STUDIES ON CHEMICAL SHIFTS OF 3,6 DIIODO 9 ETHYL 9H CARBAZOLE
 THEORETICAL STUDIES ON CHEMICAL SHIFTS OF 3,6 DIIODO 9 ETHYL 9H CARBAZOLE Teobald Kupkaa, Klaudia Radula-Janika, Krzysztof Ejsmonta, Zdzisław Daszkiewicza, Stephan P. A. Sauerb a Faculty of Chemistry,
THEORETICAL STUDIES ON CHEMICAL SHIFTS OF 3,6 DIIODO 9 ETHYL 9H CARBAZOLE Teobald Kupkaa, Klaudia Radula-Janika, Krzysztof Ejsmonta, Zdzisław Daszkiewicza, Stephan P. A. Sauerb a Faculty of Chemistry,
DOI: / /32/37
 . 2015. 4 (32) 1:18 DOI: 10.17223/1998863 /32/37 -,,. - -. :,,,,., -, -.,.-.,.,.,. -., -,.,,., -, 70 80. (.,.,. ),, -,.,, -,, (1886 1980).,.,, (.,.,..), -, -,,,, ; -, - 346, -,.. :, -, -,,,,,.,,, -,,,
. 2015. 4 (32) 1:18 DOI: 10.17223/1998863 /32/37 -,,. - -. :,,,,., -, -.,.-.,.,.,. -., -,.,,., -, 70 80. (.,.,. ),, -,.,, -,, (1886 1980).,.,, (.,.,..), -, -,,,, ; -, - 346, -,.. :, -, -,,,,,.,,, -,,,
Machine Learning for Data Science (CS4786) Lecture11. Random Projections & Canonical Correlation Analysis
 Machine Learning for Data Science (CS4786) Lecture11 5 Random Projections & Canonical Correlation Analysis The Tall, THE FAT AND THE UGLY n X d The Tall, THE FAT AND THE UGLY d X > n X d n = n d d The
Machine Learning for Data Science (CS4786) Lecture11 5 Random Projections & Canonical Correlation Analysis The Tall, THE FAT AND THE UGLY n X d The Tall, THE FAT AND THE UGLY d X > n X d n = n d d The
Zmiany techniczne wprowadzone w wersji Comarch ERP Altum
 Zmiany techniczne wprowadzone w wersji 2018.2 Copyright 2016 COMARCH SA Wszelkie prawa zastrzeżone Nieautoryzowane rozpowszechnianie całości lub fragmentu niniejszej publikacji w jakiejkolwiek postaci
Zmiany techniczne wprowadzone w wersji 2018.2 Copyright 2016 COMARCH SA Wszelkie prawa zastrzeżone Nieautoryzowane rozpowszechnianie całości lub fragmentu niniejszej publikacji w jakiejkolwiek postaci
HOW MASSIVE ARE PROTOPLANETARY/ PLANET HOSTING/PLANET FORMING DISCS?
 GREAT BARRIERS IN PLANET FORMATION, PALM COVE 26/07/2019 HOW MASSIVE ARE PROTOPLANETARY/ PLANET HOSTING/PLANET FORMING DISCS? CAN ALL THESE STRUCTURES TELL US SOMETHING ABOUT THE (GAS) DISC MASS? BENEDETTA
GREAT BARRIERS IN PLANET FORMATION, PALM COVE 26/07/2019 HOW MASSIVE ARE PROTOPLANETARY/ PLANET HOSTING/PLANET FORMING DISCS? CAN ALL THESE STRUCTURES TELL US SOMETHING ABOUT THE (GAS) DISC MASS? BENEDETTA
THE EFFECT OF THE METHOD OF POURING LIQUID METAL INTO MOULD ON MISRUN FORMATION IN THE CASTINGS MADE WITH LOST-WAX CASTING TECHNOLOGY
 A R C H I V E S O F M E C H A N I C A L T E C H N O L O G Y A N D A U T O M A T I O N Vol. 33 no. 4 2013 KRZYSZTOF GRZE KOWIAK, MARCIN WOJCIECHOWSKI KAMIL SYTEK THE EFFECT OF THE METHOD OF POURING LIQUID
A R C H I V E S O F M E C H A N I C A L T E C H N O L O G Y A N D A U T O M A T I O N Vol. 33 no. 4 2013 KRZYSZTOF GRZE KOWIAK, MARCIN WOJCIECHOWSKI KAMIL SYTEK THE EFFECT OF THE METHOD OF POURING LIQUID
What our clients think about us? A summary od survey results
 What our clients think about us? A summary od survey results customer satisfaction survey We conducted our audit in June 2015 This is the first survey about customer satisfaction Why? To get customer feedback
What our clients think about us? A summary od survey results customer satisfaction survey We conducted our audit in June 2015 This is the first survey about customer satisfaction Why? To get customer feedback
Outline of a method for fatigue life determination for selected aircraft s elements
 Outline TRIBOLOGY of a method for fatigue life determination for selected aircraft s elements 71 SCIENTIFIC PROBLEMS OF MACHINES OPERATION AND MAINTENANCE 4 (164) 2010 HENRYK TOMASZEK *, MICHAŁ JASZTAL
Outline TRIBOLOGY of a method for fatigue life determination for selected aircraft s elements 71 SCIENTIFIC PROBLEMS OF MACHINES OPERATION AND MAINTENANCE 4 (164) 2010 HENRYK TOMASZEK *, MICHAŁ JASZTAL
Streszczenie rozprawy doktorskiej
 Doskonalenie pomiaru zawartości wody w produktach spożywczych z wykorzystaniem metody wagosuszarkowej bazującej na promieniowaniu IR mgr Sławomir Janas Streszczenie rozprawy doktorskiej Promotor pracy:
Doskonalenie pomiaru zawartości wody w produktach spożywczych z wykorzystaniem metody wagosuszarkowej bazującej na promieniowaniu IR mgr Sławomir Janas Streszczenie rozprawy doktorskiej Promotor pracy:
EPS. Erasmus Policy Statement
 Wyższa Szkoła Biznesu i Przedsiębiorczości Ostrowiec Świętokrzyski College of Business and Entrepreneurship EPS Erasmus Policy Statement Deklaracja Polityki Erasmusa 2014-2020 EN The institution is located
Wyższa Szkoła Biznesu i Przedsiębiorczości Ostrowiec Świętokrzyski College of Business and Entrepreneurship EPS Erasmus Policy Statement Deklaracja Polityki Erasmusa 2014-2020 EN The institution is located
Pielgrzymka do Ojczyzny: Przemowienia i homilie Ojca Swietego Jana Pawla II (Jan Pawel II-- pierwszy Polak na Stolicy Piotrowej) (Polish Edition)
 Pielgrzymka do Ojczyzny: Przemowienia i homilie Ojca Swietego Jana Pawla II (Jan Pawel II-- pierwszy Polak na Stolicy Piotrowej) (Polish Edition) Click here if your download doesn"t start automatically
Pielgrzymka do Ojczyzny: Przemowienia i homilie Ojca Swietego Jana Pawla II (Jan Pawel II-- pierwszy Polak na Stolicy Piotrowej) (Polish Edition) Click here if your download doesn"t start automatically
Extraclass. Football Men. Season 2009/10 - Autumn round
 Extraclass Football Men Season 2009/10 - Autumn round Invitation Dear All, On the date of 29th July starts the new season of Polish Extraclass. There will be live coverage form all the matches on Canal+
Extraclass Football Men Season 2009/10 - Autumn round Invitation Dear All, On the date of 29th July starts the new season of Polish Extraclass. There will be live coverage form all the matches on Canal+
ARNOLD. EDUKACJA KULTURYSTY (POLSKA WERSJA JEZYKOWA) BY DOUGLAS KENT HALL
 Read Online and Download Ebook ARNOLD. EDUKACJA KULTURYSTY (POLSKA WERSJA JEZYKOWA) BY DOUGLAS KENT HALL DOWNLOAD EBOOK : ARNOLD. EDUKACJA KULTURYSTY (POLSKA WERSJA Click link bellow and free register
Read Online and Download Ebook ARNOLD. EDUKACJA KULTURYSTY (POLSKA WERSJA JEZYKOWA) BY DOUGLAS KENT HALL DOWNLOAD EBOOK : ARNOLD. EDUKACJA KULTURYSTY (POLSKA WERSJA Click link bellow and free register
Pro-tumoral immune cell alterations in wild type and Shbdeficient mice in response to 4T1 breast carcinomas
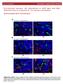 www.oncotarget.com Oncotarget, Supplementary Materials Pro-tumoral immune cell alterations in wild type and Shbdeficient mice in response to 4T1 breast carcinomas SUPPLEMENTARY MATERIALS Supplementary
www.oncotarget.com Oncotarget, Supplementary Materials Pro-tumoral immune cell alterations in wild type and Shbdeficient mice in response to 4T1 breast carcinomas SUPPLEMENTARY MATERIALS Supplementary
Financial support for start-uppres. Where to get money? - Equity. - Credit. - Local Labor Office - Six times the national average wage (22000 zł)
 Financial support for start-uppres Where to get money? - Equity - Credit - Local Labor Office - Six times the national average wage (22000 zł) - only for unymployed people - the company must operate minimum
Financial support for start-uppres Where to get money? - Equity - Credit - Local Labor Office - Six times the national average wage (22000 zł) - only for unymployed people - the company must operate minimum
TECHNOLOGIE OCHRONY ŚRODOWISKA (studia I stopnia) Derywatyzacja w analizie środowiskowej zanieczyszczeń typu jony metali i jony metaloorganiczne
 Destylacja z parą wodną jako metoda wzbogacania i izolacji zanieczyszczeń organicznych z próbek wodnych i stałych w środowiskowej analizie chromatograficznej Destylacja z parą wodną może być stosowana
Destylacja z parą wodną jako metoda wzbogacania i izolacji zanieczyszczeń organicznych z próbek wodnych i stałych w środowiskowej analizie chromatograficznej Destylacja z parą wodną może być stosowana
Katowice, plan miasta: Skala 1: = City map = Stadtplan (Polish Edition)
 Katowice, plan miasta: Skala 1:20 000 = City map = Stadtplan (Polish Edition) Polskie Przedsiebiorstwo Wydawnictw Kartograficznych im. Eugeniusza Romera Click here if your download doesn"t start automatically
Katowice, plan miasta: Skala 1:20 000 = City map = Stadtplan (Polish Edition) Polskie Przedsiebiorstwo Wydawnictw Kartograficznych im. Eugeniusza Romera Click here if your download doesn"t start automatically
Instrukcja obsługi User s manual
 Instrukcja obsługi User s manual Konfigurator Lanberg Lanberg Configurator E-mail: support@lanberg.pl support@lanberg.eu www.lanberg.pl www.lanberg.eu Lanberg 2015-2018 WERSJA VERSION: 2018/11 Instrukcja
Instrukcja obsługi User s manual Konfigurator Lanberg Lanberg Configurator E-mail: support@lanberg.pl support@lanberg.eu www.lanberg.pl www.lanberg.eu Lanberg 2015-2018 WERSJA VERSION: 2018/11 Instrukcja
Cracow University of Economics Poland
 Cracow University of Economics Poland Sources of Real GDP per Capita Growth: Polish Regional-Macroeconomic Dimensions 2000-2005 - Keynote Speech - Presented by: Dr. David Clowes The Growth Research Unit,
Cracow University of Economics Poland Sources of Real GDP per Capita Growth: Polish Regional-Macroeconomic Dimensions 2000-2005 - Keynote Speech - Presented by: Dr. David Clowes The Growth Research Unit,
KONSPEKT DO LEKCJI MATEMATYKI W KLASIE 3 POLO/ A LAYER FOR CLASS 3 POLO MATHEMATICS
 KONSPEKT DO LEKCJI MATEMATYKI W KLASIE 3 POLO/ A LAYER FOR CLASS 3 POLO MATHEMATICS Temat: Funkcja logarytmiczna (i wykładnicza)/ Logarithmic (and exponential) function Typ lekcji: Lekcja ćwiczeniowa/training
KONSPEKT DO LEKCJI MATEMATYKI W KLASIE 3 POLO/ A LAYER FOR CLASS 3 POLO MATHEMATICS Temat: Funkcja logarytmiczna (i wykładnicza)/ Logarithmic (and exponential) function Typ lekcji: Lekcja ćwiczeniowa/training
PROCEEDINGS OF THE INSTITUTE OF VEHICLES 2(106)/2016 (12 pt)
 PROCEEDINGS OF THE INSTITUTE OF VEHICLES 2(106)/2016 (12 pt) (12 pt) Henryk Bąkowski 1, Zbigniew Stanik 2 (12 pt) THE TEST OF THE COOLING FLUID WEAR USED IN MOTOR VEHICLES (12 pt) 1. Introduction Cooling
PROCEEDINGS OF THE INSTITUTE OF VEHICLES 2(106)/2016 (12 pt) (12 pt) Henryk Bąkowski 1, Zbigniew Stanik 2 (12 pt) THE TEST OF THE COOLING FLUID WEAR USED IN MOTOR VEHICLES (12 pt) 1. Introduction Cooling
Unit of Social Gerontology, Institute of Labour and Social Studies ageing and its consequences for society
 Prof. Piotr Bledowski, Ph.D. Institute of Social Economy, Warsaw School of Economics local policy, social security, labour market Unit of Social Gerontology, Institute of Labour and Social Studies ageing
Prof. Piotr Bledowski, Ph.D. Institute of Social Economy, Warsaw School of Economics local policy, social security, labour market Unit of Social Gerontology, Institute of Labour and Social Studies ageing
Ankiety Nowe funkcje! Pomoc magda.szewczyk@slo-wroc.pl. magda.szewczyk@slo-wroc.pl. Twoje konto Wyloguj. BIODIVERSITY OF RIVERS: Survey to students
 Ankiety Nowe funkcje! Pomoc magda.szewczyk@slo-wroc.pl Back Twoje konto Wyloguj magda.szewczyk@slo-wroc.pl BIODIVERSITY OF RIVERS: Survey to students Tworzenie ankiety Udostępnianie Analiza (55) Wyniki
Ankiety Nowe funkcje! Pomoc magda.szewczyk@slo-wroc.pl Back Twoje konto Wyloguj magda.szewczyk@slo-wroc.pl BIODIVERSITY OF RIVERS: Survey to students Tworzenie ankiety Udostępnianie Analiza (55) Wyniki
Equipment for ultrasound disintegration of sewage sludge disseminated within the Record Biomap project (Horizon 2020)
 Research Coordination for a Low-Cost Biomethane Production at Small and Medium Scale Applications, akronim Record Biomap Equipment for ultrasound disintegration of sewage sludge disseminated within the
Research Coordination for a Low-Cost Biomethane Production at Small and Medium Scale Applications, akronim Record Biomap Equipment for ultrasound disintegration of sewage sludge disseminated within the
ORGANICZNE ZWI ZKI SIARKI JAKO INICJATORY I KOINICJATORY FOTOPOLIMERYZACJI RODNIKOWEJ
 AKADEMIA TECHNICZNO-ROLNICZA IM. JANA I JÊDRZEJA ŒNIADECKICH W BYDGOSZCZY ROZPRAWY NR 112 Andrzej Wrzyszczyñski ORGANICZNE ZWI ZKI SIARKI JAKO INICJATORY I KOINICJATORY FOTOPOLIMERYZACJI RODNIKOWEJ BYDGOSZCZ
AKADEMIA TECHNICZNO-ROLNICZA IM. JANA I JÊDRZEJA ŒNIADECKICH W BYDGOSZCZY ROZPRAWY NR 112 Andrzej Wrzyszczyñski ORGANICZNE ZWI ZKI SIARKI JAKO INICJATORY I KOINICJATORY FOTOPOLIMERYZACJI RODNIKOWEJ BYDGOSZCZ
ELECTRIC AND MAGNETIC FIELDS NEAR NEW POWER TRANSMISSION LINES POLA ELEKTRYCZNE I MAGNETYCZNE WOKÓŁ NOWYCH LINII ELEKTROENERGETYCZNYCH
 ELEKTRYKA 2012 Zeszyt 3-4 (223-224) Rok LVIII Olgierd MAŁYSZKO, Michał ZEŃCZAK Katedra Elektroenergetyki i Napędów Elektrycznych, Zachodniopomorski Uniwersytet Technologiczny w Szczecinie w Szczecinie
ELEKTRYKA 2012 Zeszyt 3-4 (223-224) Rok LVIII Olgierd MAŁYSZKO, Michał ZEŃCZAK Katedra Elektroenergetyki i Napędów Elektrycznych, Zachodniopomorski Uniwersytet Technologiczny w Szczecinie w Szczecinie
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume Issue 1 DOI: /amm
 A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 59 2014 Issue 1 DOI: 10.2478/amm-2014-0056 T. KNYCH, M. PIWOWARSKA-ULIASZ, P. ULIASZ ALUMINIUM ALLOYS WITH ZIRCONIUM ADDITIONS, IN
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 59 2014 Issue 1 DOI: 10.2478/amm-2014-0056 T. KNYCH, M. PIWOWARSKA-ULIASZ, P. ULIASZ ALUMINIUM ALLOYS WITH ZIRCONIUM ADDITIONS, IN
