W. GUMOWSKA, I. DOBOSZ, M. UHLEMANN, J. KOZA. Al 2 O 3 Co AND Al 2 O 3 Fe COMPOSITES OBTAINED BY THE ELECTROCHEMICAL METHOD
|
|
|
- Antonina Adamczyk
- 7 lat temu
- Przeglądów:
Transkrypt
1 A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume Issue 4 W. GUMOWSKA, I. DOBOSZ, M. UHLEMANN, J. KOZA Al 2 O 3 Co AND Al 2 O 3 Fe COMPOSITES OBTAINED BY THE ELECTROCHEMICAL METHOD OTRZYMYWANIE KOMPOZYTÓW Al 2 O 3 Co i Al 2 O 3 Fe METODĄ ELEKTROCHEMICZNĄ The electrochemical method for obtaining the oxide coatings of the Al-Al 2 O 3 -M composite structure is based on a two-stage process: anodic oxidation of aluminium with the formation of a porous oxide coating cathodic deposition of foreign metal ions (e.g. Ni, Co, Fe, Cu, Ag, etc.) from an aqueous solution with the direct, pulsating, reversing, or alternating current. In the present study, the oxide layers on aluminium with an ordered pore structure were obtained in a two-stage process of anodic oxidation in an oxalic acid solution. In the Al 2 O 3 layers that are obtained, the cell parameters were changed by pore widening and barrier layer thickness reduction. These processes resulted in obtaining thin membranes, in which cells in the aluminium oxide layer had the following dimensions: pore diameter of 80 nm, single pore wall thickness of 25 nm, and a height of 5 µm. Those membranes were used as a templates for the incorporation of cobalt and iron particles. The metals were deposited in to membrane pores by the electrodeposition of cobalt and iron from sulphate electrolytes. Measurements were carried out using direct and reversing current (PRC). The microscopic observations of cobalt and iron nanowires indicate that the membrane pores were not entirely filled by deposited metals. Although in practice, each pore was built-up by a metal, but the length of the nanowires ranged from 1 (Co) to 2 µm (Fe), while the pore height in the membrane was approx. 5 µm. The cathode efficiencies of the studied metals electrodeposition were determined by using direct current. These efficiencies were not high due to hydrogen co-evolution. Metoda elektrochemiczna otrzymywania powłok tlenkowych o budowie kompozytowej Al-Al 2 O 3 -M bazuje na procesie dwuetapowym : anodowe utlenianie aluminium z wytworzeniem porowatej powłoki tlenkowej katodowa redukcja jonów obcego metalu (np.ni, Co, Fe, Cu, Ag itp. ) z zastosowaniem prądu stałego, pulsacyjnego, rewersyjnego lub zmiennego. W przedstawianej pracy otrzymywano warstewki tlenkowe na aluminium, o uporządkowanej strukturze porów, w dwuetapowym procesie anodowego utleniania w roztworze kwasu szczawiowego. W otrzymanych warstewkach Al 2 O 3 zmieniano parametry komórek przez poszerzenie porów oraz zmniejszenie grubości warstwy zaporowej. W wyniku tych procesów uzyskano cienkie membrany, w których komórki w warstewce tlenku glinu miały następujące wymiary: średnica porów 80 nm, grubość ścianek 25 nm, a ich wysokość 5 µm. Membrany te stanowiły szablon dla wbudowywanych metali : kobaltu i żelaza. Metale osadzono w porach membrany w procesie katodowej redukcji jonów Co 2+ i Fe 2+ z elektrolitów siarczanowych. Pomiary prowadzono z zastosowaniem prądu stałego i rewersyjnego (PRC). Obserwacje mikroskopowe uzyskanych nanodrutów kobaltu i żelaza wskazują, że w żadnym przypadku pory membrany nie były wypełnione całkowicie przez osadzane metale. Wprawdzie praktycznie każdy por został zabudowany przez metal, jednak długość otrzymanych nanodrutów wynosiła od 1 (Co) do 2 µm (Fe), podczas gdy wysokość porów w wytworzonej membranie wynosiła około 5 µm. Wyznaczono katodowe wydajności prądowe elektroosadzania badanych metali z zastosowaniem prądu stałego. Wydajności te nie są zbyt wysokie ze względu na współwydzielanie wodoru. AGH UNIVERSITY OF SCIENCE AND TECHNOLOGY, FACULTY OF NON-FERROUS METALS, KRAKÓW, 30. MICKIEWICZA AV., POLAND LEIBNIZ INSTITUT FÜR FESTKÖRPER UND WERKSTOFFFORSCHUNG DRESDEN
2 Introduction Aluminium is a chemically active metal, with a high affinity to oxygen, and despite that it is still resistant to the corrosive action of numerous environments and a number of chemical reagents. Aluminium showes its resistance due to the protective nature of the oxide layer on the metal s surface. The porous and ordered structure of the oxide layers obtained on aluminium in the process of anodic oxidation is especially appropriate to production of Al- Al 2 O 3 -M (M other metal) composite layers. The oxide layers on aluminium are most often produced in the process of anodic oxidation in the sulphuric, oxalic, phosphoric, or chromic acids solutions [1-11]. The following electrode reactions occur in the process by using a direct current : Al Al e, at the anode (1) 2H + + 2e H 2, at the cathode (2) The layer of aluminium oxide forms in the reaction of Al 3+ ions with O 2 ions: 6OH 3H 2 O + 3O 2 (3) 2Al O 2 Al 2 O 3 (4) Porous alumina layer forms in consecutive stages: formation of a thin ( µm) oxide layer, the so-called blocking or barrier layer transformation of the barrier layer on the boundary of phases: oxide electrolyte (formation of a porous layer) increase in the porous layer thickness (from a few to a few dozen µm) The alumina layer characterizes with ordered structure in the form of hexagonal close-packed honeycomb type cells. In each of them are centrally located pores, separated from the metal surface by a thin, so-called barrier layer. Fig. 1 shows the schematic oxide layer structure (a), ordered cells structure, (b) and the structure and dimensions of a single cell (c). The pore and cell dimensions as well as the barrier layer thickness are depend on the voltage applied in the process of anodic aluminium oxidation; they also depend on the electrolyte type, composition, and temperature [12-15]. In their studies Keller et al. [12] described in detail the results referring to the relation between individual cells dimensions and the anodizing conditions. Composition of electrolytes and process parameters are shown in Table 1. a) b) c) A Al 2 O 3 Al B Fig. 1. Diagram of the porous structure of the oxide layer obtained in the process of anodic oxidation of aluminium. a. A porous layer, B barrier layer b. ordered hexagonal structure of the porous layer c. structure and dimensions of a single cell of the oxide layer that was obtained in the process of anodic oxidation of aluminium in a 4% solution of phosphoric acid at a voltage of 120 V, [12]
3 1121 Conditions of anodic oxidation of aluminium [12] Electrolyte Temperature, Electrolysis Cells number, t voltage, U N N 10 C V 9 cm % sulphuric acid % oxalic acid % chromic acid % phosphoric acid TABLE 1 The data in the table show that the cells (and hence the pores) number changes in a relatively wide range, from to (per 1 cm 2 of surface). This depends on the type of electrolyte and on the voltage applied in the process. At the same time, the pores dimensions were seen to change, namely: the smallest in diameter (120 Å) pores were obtained in the sulphuric acid solutions, while in phosphoric acids solutions, the pores diameter was the largest, equal to 330 Å. Extensive investigations on formation and the methods for creating an appropriately ordered structure of the Al 2 O 3 layer [16-21] have increased interest in this system in recent years. Al-Al 2 O 3 composite is a material of special applications in the processes of obtaining nanomaterials based on a porous and ordered structure of the oxide layer on aluminium, which works as a template to incorporation specific materials in its pores. In this way the nanowires of metals, metal alloys, or multilayer Al 2 O 3 metal (alloy) composite systems of the reproduced structure of the membrane used are obtained (replica method) [8-12, 16-24]. Thin layers become especially interesting for information technologies (IT) for high density magnetic recording systems. They are used for production of minidisks, hard disks, video players and recorders, in personal computers and car navigation systems. Because of the demand for such equipment with increasingly high speed of information recording, the requirements related to the magnetic flux density have been sharply increased. Since 2002 this amount increased from 150 Gbit/inch 2 (23 Gbit/cm 2 ) to 200 Gbit/inch 2 (31 Gbit/cm 2 ) and even to 1 Tbit/inch 2 (0.155 Tbit/cm 2 ) [18]. Magnetic materials for information carriers are based on layers with high magnetic anisotropy in the perpendicular direction to the layer surface. For the first time a perpendicular system of recording was suggested by Iwasaki [25]. The electrodeposition of cobalt or its alloys (e.g. CoFe, CoNi, CoNiFe, etc.) nanowires in aluminium oxide pores is most frequently used to obtain magnetic information carriers [19, 20, 26]. The basic stages of the process resulting in the obtaining of nanomaterials in Al 2 O 3 layer pores comprise: appropriate preparation of an Al 2 O 3 matrix in the form of a membrane selection of parameters of metal or alloy electrodeposition in the pores. It has been shown that an oxide layer on aluminium with an high ordered structure forms as a result of two-stage anodising [5, 17, 21, 27, 28]. A detailed description of this method was presented by Nielsch [17]. The first stage of the process consists of a long-lasting (a few dozen hours) anodic oxidation of aluminium at a voltage of 40V, which leads to the typical hexagonal cell structure of aluminium oxide. A 3% solution of oxalic acid is used as the electrolyte at a temperature of 2 C. As the aluminium oxide layer after this process features non-uniform pore distribution, it is chemically removed in a phosphoric acid solution or in a mixture of phosphoric and chromic acids. Once this layer is removed, a hexagonal pore structure remains on the aluminium surface, which is the template for the uniform hexagonal structure of the layer forming in the second stage of anodic oxidation. The second anodizing stage is relatively short (a few hours) and proceeds in a few steps: anodic oxidation, chemical pore widening, and barrier layer thinning. The dependence of electrolysis voltage and current density on the anodizing time is recorded during this process and some anodizing parameters are gradually changed. Figure 2 shows the schematic course of the consecutive parts of the second anodizing stage (I) and corresponding changes of the oxide layer structure (II). Dotted lines also mark the symbolic stages before (a) and after (e) the second stage of anodic aluminium oxidation to enable a comparison of the differences occurring in the layer structure. The relationships in the figure 2 show that a minimum of current density is observed at the beginning period of the second stage of anodic oxidation, corresponding to the barrier layer formation. During the continued oxidation, up to an hour approx., the rate of layer formation stabilises (the rate of aluminium oxide formation is equal to the rate of its dissolution by the electrolyte), and as a result the barrier layer thickness stabilises. Further treatment of the alumina membrane leads to the reduction of the barrier layer thickness. It is carried out by the further oxidation in the same solution at a
4 1122 I a) b) c) d) e) temperature of approx. 30 C. Under those conditions, the rate of layer dissolution is higher than the rate of its formation, which leads to pore widening and barrier layer thinning. The process is accompanied by decreasing electrolysis voltage, which after 4 hours decreases to 25 V. II current densityy, ma/cm 2 voltage ,5 2,0 1,5 1,0 0, ,5 1,0 4,0 4,5 time, h 0 0,5 1,0 4,0 4,5 time, h // // Al porous alumina barrier layer Al porous alumina with electrodeposited metal Al Fig. 2. The course of voltage and current density versus time in the process of anodic oxidation of aluminium (I) and the structure of the layer formed in each of the steps (II) [17]: a. the first anodizing step long-lasting anodic oxidation in a 3% solution of oxalic acid, at 2 C, and removal of the oxide layer of an unordered structure b. the second anodizing step obtaining an ordered oxide structure c. pore widening (t = 30 C) with a simultaneous barrier layer thinning d. further reduction of the barrier layer thickness e. foreign metal electrodeposition in membrane pores The next step consists of breaking the process of pore widening, which is achieved by cooling the electrolyte to 2 C. Then, for further reduction of the barrier layer thickness, lower current densities are used during the anodic oxidation than that at the initial stage. The final effect of the described preparations of an Al-Al 2 O 3 membrane is the reduction of the barrier layer thickness to 10 nm. At the same time, the electrolysis voltage in the studied system reduces to 6-7 V. Careful preparation of an aluminium oxide matrix guarantees that Al-Al 2 O 3 -Metal (or alloy) composites featuring a uniform and densely-packed structure are obtained in the process of foreign metal electrodeposition in the oxide layer pores. This process is carried out using direct, alternating, pulsating, or reversing current. As the application of direct current usually results in non-uniform pore build-up, most of these composites are obtained by using alternating, pulsating or reversing current [17, 22]. During the flow of alternating or reversing current, in cathodic periods of current flow, the incorporation metal ions are reduced according to the reaction: M n+ + ne M (5) which is accompanied by hydrogen co-evolution (reaction 2).
5 1123 The hydrogen co-evolution is an unfavourable process: it causes a decrease in the cathodic efficiency of metal electrodeposition gaseous hydrogen may block the pores in the layer cathodic reduction of hydrogen ions results in a ph increase electrolyte alkalisation occurs close to the cathode surface, which may cause metal hydroxide precipitation and even damage to the porous ordered structure of the alumina layer. The application of pulsating or reversing current hinders out those unfavourable effects in the periods of reverse current flow to a large extent. It should be emphasised that the production of the nanomaterials requires individual techniques, depending on the type of composite. The barrier layer is very often removed, which leads to a structure with pores open on both sides. The process of gold nanowires, based on an Al 2 O 3 membrane, can be used as an example (Fig. 3 [24]). A pore Al 2 O 3 membrane B Sputtered Ag film Electrodeposited Ag C D Electrodeposited Au Au nanoparticle os Fig. 3. Diagram of gold nano-wires production In the first step (A) silver was sputtered on an Al 2 O 3 matrix surface and afterwards: silver (B) and then gold (C) were consecutively electrodeposited. The final step was the removal of the silver with nitric acid (D). Nanocomposites that are based on the ordered structure of an oxide layer on aluminium: Al-Al 2 O 3 -Metal (or alloy) and Al 2 O 3 -Metal (or alloy) constitute a group of modern materials characterized with special properties and wide range of applications. In the present study, cobalt and iron nanowires were obtained in the process of those metals electrodeposition in the pores of an alumina membrane on a copper plate. 2. Experimental 2.1. Materials The specimens were aluminium of the composition: Al min %; Cu 0.3 ppm; Fe 0.3 ppm; Mg 1.2 ppm; Si 0.8 ppm (Goodfellow). The specimen s surface was 0.8 cm 2 and 0.3 mm thick. Before the measurements were performed, the specimens were degreased in acetone for 3 minutes, with the application of ultrasound. Then the specimens were annealed in an argon atmosphere for 3 hours, at 500 C, to remove any residual stress and for homogenization of its composition. The annealed specimens were electropolished (Stuers LectroPol-5 electropolisher) in a mixture of HClO 4 and C 2 H 5 OH solutions with the addition of butyl cellulose. Table 3 shows the parameters of electropolishing.
6 1124 Parameters of electropolishing Temperature 16 C Time 20 s Voltage 48 V Electrolyte flow 16 cm 3 /min Electrolyte 78 cm 3 HClO 4 composition 700 cm 3 C 2 H 5 OH 100 cm 3 butyl cellulose 120 cm 3 H 2 O 2.2. Anodic oxidation of aluminium TABLE 2 The anodic oxidation process was carried out in two steps. 0.3 M oxalic acid was used in both of them. The first step was carried out at constant electrolysis voltage of 40V and 2 C. Low temperature is needed during anodizing steps because temperatures higher then 5 C may cause thermal shocks and breakdowns in the barrier layer [17]. The schematic diagram of the experimental apparatus is shown in Fig. 4. The electrolyser (made of Teflon) had an opening in the bottom, in which the Al anode was fixed. One surface of the specimen was in contact with the electrolyte, the other external with a copper plate, which ensured the current connection to the aluminium electrode. The cathode was made of aluminium and placed parallel to the specimen. To ensure low temperatures, the system was cooled using a cryostat. Temperature of electrolyte was measured continuously. The electrolyte was stirred by means of a mechanical stirrer. Fig. 4. Diagram of the experimental set. 1 electrolyser, 2 Al anode, 3 Al cathode, 4 electrolyte, 5 Cu block, 6 aluminium block, 7 pipe with KRYO 30 cooling liquid, 8 cryostat, 9 temperature controller, 10 stirrer, 11 stabilised power supply, 12 data recording system (KEITLEY 199) The time of the first anodising step was 24 hours. The oxide layer that was obtained in this step was removed in a solution containing H 3 PO 4 (35 cm 3 /dm 3 ) and CrO 3 (20g/dm 3 ) at 80 C for 2 hours. After the oxide layer removal, the specimens were anodic oxidised (step two) for 5 hours. Then the specimens were subjected to pore widening by heating the oxalic acid up to 40 C for 2 hours. In the next step, which aimed at the thinning of the barrier layer, the anodizing was continued, during which the electrolysis voltage was gradually reduced from 10.1 to 5.0 V. These parameters are shown in Table 3. Parameters of the thinning process of the barrier layer Anodizing voltage, V Time, min TABLE 3
7 Aluminium and barrier layer removal The aluminium substrate was dissolved after two anodizing steps. This process was carried out in a 1:3 mixture of HCl and CuCl 2 solutions (100 cm 3 of 38%HCl + 3,4 g of CuCl 2 H 2 O cm 3 H 2 O), at room temperature for approx minutes. In these conditions the aluminium react with CuCl 2 solution: 2.5. Cathodic deposition of metals The process of cobalt and iron incorporation in to the membrane pores was carried out in the electrodeposition of Co 2+ and Fe 2+ ions from sulphate baths, applying direct or reversing current (PRC). Electrodeposition was carried out using IPS AJ potentiostat at room temperature. The composition of electrolytes is shown in Table 4. 2Al + 3Cu 2+ 2Al 3+ 3Cu (6) Composition of electrolytes for Co and Fe deposition TABLE 4 After this process, the pores opened. The barrier layer was etched in a 5% H 3 PO 4 solution at 30 C for 15 minutes Substrate deposition After removal of the aluminium and barrier layer, a thin layer of gold was sputter on the fracture of the template (on the open pores of the external specimen s surface).then, copper was electrolytically deposited from a sulphate bath. The composition of electrolyte was: 65g CuSO 4, 184g H 2 SO 4 and 105 mg KCl in dm 3. The electrolysis was carried out using direct current, under potentiostatic conditions at potential -300 mv (vs SCE); the time of electrolysis 10 minutes. IPS AJ potentiostat was used in the measurements. In this way the porous membranes were obtained with the structure, which is shown in the diagram in Fig. 5. Al 2 O 3 pore Electrolysis Electrolyte composition ph Cobalt deposition Iron deposition g/dm 3 CoSO 4.7H 2 O 25 g/dm 3 H 3 BO g/dm 3 FeSO 4.7H 2 O 25 g/dm 3 H 3 BO In the process of metal electrodeposition, the specimen (membrane) was placed horizontally in the electrolyser made of Teflon. A platinum sheet was an auxiliary electrode, while the saturated calomel electrode was the reference electrode. Analytical purity reagents were used to prepare the solutions. The ph value was controlled by the addition of a 10% H 2 SO 4 or NaOH solution. Electrodeposition was carried out using direct current, under potentiostatic conditions (E = -1.2 V (vs. SCE)), or by using reversing current (PRC). IPS AJ potentiostat was used in the measurements. The measurements under PRC conditions were performed by applying: the time of cathodic current flow t K = 140 ms; potential E K = V (vs. SCE); the time of anodic direction of the current flow t A = 20 ms; potentials: E A = 0 V (vs. SCE) for Co; E A = -0,350 V (vs. SCE) for Fe. Selected materials were subjected to observations on a scanning electron microscope (FE Gemini LEO 1530). Au Fig. 5. Diagram of the alumina membrane structure Cu Sputtering gold is aimed at the closing pores, while the layer of electrodeposited copper causes a reduction of electrolysis voltage in the process of metal electrodeposition in the pores of an Al 2 O 3 layer. 3. Results and discussion 3.1. Membrane preparation Membrane preparation is an important step in the nanowire deposition. Depending on the anodizing parameters (voltage, time) applied, we can control the pore diameter and height. During step I of anodic oxidation of aluminium, the relationship between current and time was recorded. The course of such a relationship is shown in Figure 6.
8 1126 Fig. 6. Current density versus time during the first step of anodizing A sudden decrease in the current intensity was observed in the initial stage of the process, which is related to the beginning of the oxide barrier layer formation. After a few minutes, the rate of the layer formation is equalised with the rate of its dissolution by the electrolyte a constant current intensity is observed. Pores start growing in various directions and pores distribution in the oxide is entirely irregular. Therefore, after a long-lasting period of the first anodising step the porous unordered oxide structure was removed in a solution containing H 3 PO 4 (35 cm 3 /dm 3 ) and CrO 3 (20g/dm 3 ) at 80 C for 2 hours. Cavities remain after oxide removal, which make the template for future pores formation. After this process, the specimen was subjected to repeated anodic oxidation (step II). Fig. 7 shows the dependence of the anodic current density on oxidation time. Fig. 7. Current density versus time during the second step of anodizing
9 1127 As a result of the second anodizing step, the pores grow perpendicular to the surface and form an ordered hexagonal structure, which was confirmed by scanning microscope observations (Fig. 8). Fig. 8. Microstructure of aluminium specimen after the second anodizing stage Pore dimensions and the porosity of the membrane are shown in Table 5. TABLE 5 Dimensions of membrane obtained Pore diameter D p nm 80 ( 0,08µm) Pore height L µm 5 Distance between the pores l nm 105 ( 0,1 µm) 3.2. Nanowire deposition Surface fraction of pores % 50 Cobalt and iron nanowires were obtained in the process of electrodeposition in the nanopores of an aluminium oxide membrane under potentiostatic conditions by using direct or reversing (PRC) current Electrodeposition of cobalt nanowires direct current applied to determine the optimal potentials for Co electrodeposition. The dependence of current density on potential i = f(e) is obtained from cyclic voltammetry. This relationship is shown in Figure 9. The potentials are given versus SCE. Based on voltammogramme, it is possible to conclude that cobalt electrodeposition occurs in the potentials that range from -0.7 V to -1.2 V. A potential was selected from this range, at which cobalt was electrodeposited in membrane pores, based on the values of cathodic current efficiency. Cathodic current efficiencies were determined from an electrode mass increase, in which the amount of charges flowing through the system was determined by planimetry of I = f(t) curve. The measurement was performed at a few potentials, which values result from the voltammetry. The relationship between the current efficiency and potential is shown in Figure 10. Cobalt was deposited from sulphate baths of the composition shown in Table 4. Cyclic voltammetry was
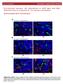
10 1128 Fig. 9. Voltammogramme for cobalt The maximum of cathodic efficiency was observed at the potential of -1.2 V. This potential was selected as the most appropriate in the measurements of cobalt electrodeposition, because it ensures the highest degree of metal incorporation into the membrane pores. 90 cathodic current efficiency, % ,2-1,1-1,0-0,9-0,8 potential E, V (vs.sce) Fig. 10. Dependence of cathodic current efficiency on the potential cobalt electrodeposition Specimens after cobalt electrodeposition were observed on a scanning microscope. The pore diameter and nanowire height was determined. Observations of the cross-section of the Al 2 O 3 Co were carried out. Fig. 11 shows the microphotograph of Co nanowires obtained during electrodeposition in potentiostatic conditions, (E = -1.2 V(vs. SCE)).
11 1129 Cu Co Al 2 O 3 Fig. 11. SEM images of template after Co electrodeposition under direct current, E = -1.2 V (vs.sce) The obtained nanowires are uniform, cobalt is deposited in each pore and the pores are around 1 µm high. On the composite surface, on Al 2 O 3 electrolyte phase boundary, a deposit of cobalt hydroxide was observed (in the picture below Al 2 O 3 ), which is not seen in the microphotograph presented. It is formed due to the ph decrease resulting from the cathodic reduction of hydrogen ions (reaction (2)) Electrodeposition of cobalt nanowires reversing current (PRC) following parameters was used in the process of electrodeposition: E K = -1.2 V (vs. SCE), t K =140 ms; E A = 0 V (vs.sce), t A = 20ms. Specimens were subjected to observations on a scanning microscope. The pore diameter and nanowire height were determined. Observations of the cross-section of the surface were carried out. Fig. 12 shows the microphotograph of Co nanowires obtained during electrodeposition under PRC conditions. Cobalt was deposited from sulphate baths of the composition shown in Table 4. Reversing current of the Al 2O 3 Co Cu Fig. 12. SEM images of template after Co electrodeposition using reversing current: E K = -1.2 V (vs. SCE), t K =140 ms; E A = 0 V(vs. SCE) t A = 20ms
12 1130 The nanowires are not uniform and differ in height. Cobalt is deposited nearly in each pore Electrodeposition of iron nanowires direct current Iron was deposited from sulphate baths of the composition shown in Table 4. Cyclic voltammetry was carried out to determine the optimal potentials for Fe electrodeposition. The relationship between the current density and potential i = f(e) is shown in Figure 13. Fig. 13. Voltammogramme for iron Based on voltammogramme it is possible to conclude that iron electrodeposition occurs in the potentials that range from -0.9 V to -1.4 V. A potential was selected from this range, at which iron was electrodeposited in the membrane pores, based on the values of cathodic efficiency. The dependence of cathodic current efficiency on the potential of cathodic Fe 2+ ions reduction is shown in Fig. 14. A maximum of cathodic current efficiency is observed at the potential of V. The cathodic current efficiencies, both for iron and cobalt electrodeposition, are not high. This is understandable, because gaseous hydrogen co-evolves apart from metal ions reduction in the cathodic process. Iron electrodeposition measurements were made at the potential of -1.2 V (vs.sce).
13 cathodic current efficiency, % ,25-1,20-1,15-1,10-1,05 potential E, V (vs.sce) -1,00 Fig. 14. Dependence of cathodic current efficiency on the potential iron electrodeposition Specimens after iron electrodeposition were subjected to observations on a scanning microscope. The pore diameter and nanowire height were determined. Observations of the cross-section of the surface were carried out. Fig. 15 shows of a microphotograph of the Fe nanowires obtained during direct current electrodeposition under potentiostatic conditions ( E = -1.2 V (vs.sce)). Al 2 O 3 Fe Cu Fig. 15. SEM images of template after Co electrodeposition under direct current, E K = -1.2 V (vs.sce) Iron was uniformly deposited in each pore. The nanowires obtained have the same height, equal to approx. 2 µm Electrodeposition of iron nanowires reversing current (PRC) Iron was deposited from sulphate baths of the composition shown in Table 4. Reversing current of the following parameters was used in the process of electrodeposition: E K = -1.2 V (vs. SCE), t K =140 ms; E A = V(vs. SCE), t A = 20ms. Specimens were subjected to observations on a scanning microscope. The pore diameter and nanowire height were determined. Observations of the cross-section of the surface were carried out. Fig. 16 shows a microphotograph of the Fe nanowires obtained during electrodeposition under the reversing current conditions. The microphotograph shows that iron is deposited in each pore. The nanowires are not uniform and differ in height.
14 1132 Cu Fe Al 2 O 3 Fe(OH) 2 Fig. 16. SEM images of template after Co electrodeposition using reversing (PRC) current: E K = -1.2 V(vs.SCE), t K =140 ms; E A = V(vs.SCE) t A = 20ms Microscopic observations of the cobalt and iron nanowires (Fig. 11, 12, 15 and 16) indicate that the membrane pores were not entirely filled by deposited metals. Although in practice each pore was fulfilled with a metal, but the length of nanowires ranged from 1 to 2 µm, while the pore height in the membrane amounted to approx. 5 µm. In the microphotograph, in the Al 2 O 3 electrolyte interface, a clear iron hydroxide deposit is seen, which forms due to a ph decrease as a result of cathodic reduction of hydrogen ions (reaction (2)). 4. Conclusions 1. Nanocomposite Al 2 O 3 Co and Al 2 O 3 Fe were obtained on a copper plate. 2. Cobalt and iron nanowires were obtained in the process of cathodic deposition of those metals ions in the pores of an aluminium oxide layer (membrane). Experiments were performed using direct or reversing (PRC) current. 3. The nanowires distribution in the pores of an aluminium oxide layer is uniform, in the processes of electrolysis using both direct and reversing current. 4. Cathodic efficiencies depend on the potential at which Co and Fe were electrodeposited. The maximum values were obtained during the process carried out at the potential of -1.2 V (vs. SCE). They amount to: 85% for cobalt, 27% for iron. Hydrogen co-evolution is the reason for the relatively low current efficiencies. Acknowledgements This research work has been supported by the Polish Ministry of Science and Higher Education under project No REFERENCES [1] D. Al. M a w l a w i, N. C o o m b s, M. M o s k o v i t s, J.Appl.Phys. 70, 70 (1991). [2] F. L i, R. M. M e t z g e r, W. D. D o y l e, IEEE Trans. Magn. 33, 375 (1947). [3] J. L i, M. M o s k o v i t s, T. L. H a s l e t, Chem.Mater 10, 1963 (1998). [4] J. S. S u h, J. S. L e e, Appl.Phys.Lett. 75, 2047 (1999). [5] J. J a s e n s k y, F. M ü l l e r, U. G ö s e l e, J.Electrochem.Soc. 145, (11), 3735 (1998). [6] H. A s o h, K. N i s h i o, M. N a k a o, T. T a k a m u - r a, H. M a s u d a, J.Electrochem.Soc. 148, (4), B 152 (2001). [7] H. M a s u d a, F. H a s e g w a, J.Electrochem.Soc. 144, (5), L 127 (1997). [8] G. S u l k a, S. S t r o o b a n t s, V. M o s h e h a l k o v, G. B o r g h s, J.-P. C e l i s, J. Electrochem. Soc. 149, (7), D 97 (2002). [9] G. S u l k a, S. S t r o o b a n t s, V. M o s h e h a l k o v, G. B o r g h s, J.-P. C e l i s, J.Electrochem. Soc. 151, (5), B 260 (2004). [10] G. S u l k a, K. G. P a r k o ł a, Thin Solid Films 515, 338 (2006). [11] G. S u l k a, K. G. P a r k o ł a, Electrochimica Acta 52, 188 (2007). [12] F. K e l l e r, M. S. H u n t e r, D. L. R o b i n s o n, J.Electrochem.Soc. 100, 411 (1953).
15 1133 [13] A. P. L i, F. M ü l l e r, A. B i r n e r, K. N i e l s c h, U. G ö s e l e, J.Appl.Phys. 84, 6023 (1998). [14] R. C. F u r n e a u x, W. R. R i g b y, A. P. D a v i d - s o n, Nature 337, 147 (1989). [15] A. J a g m i n a s, D. B i g e l i e n é, J. M i k u l s k a s, R. T o m a s i ũ n a s, J.Crystal Growth 233, 591 (2001). [16] M. J. T i r n e y, Ch. R. M a r t i n, J.Electrochem.Soc. 137 (12), 3789 (1990). [17] K. N i e l s c h, F. M ü l l e r, A. P. L i, U. G ö s e l e, Adv. Mater. 12 (8), 582 (2000). [18] T. O s a k a, T. A s a h i, J. K a w a j i, T. Y o k o s h i - m a, Electrochimica Acta 50, 4576 (2005). [19] D. H. Q i n, L. C a o, Q. Y. S u n, Y. H u a n g, H. L. L i, Chem.Phys.Latters 358, 484 (2002). [20] S. K a w a i, R. M e d a, J.Electrochem.Soc. 122 (1), 32 (1975). [21] H. M a s u d a, K. F u k u d a, Science 268, 1466 (1995). [22] R. I n g u a n t a, M. B u t e r a, C. S u n s e r i, S. P i - a z z a, Appl. Surf. Science 253, 5447 (2007). [23] A. J a g m i n i e n é, G. V a l i n č i u s, A. R i a u k a- i t é, A. J a g m i n a s, J.Crystal Growth 274, 622 (2005). [24] J. C. H u l t e n, Ch. R. M a r t i n, J.Mater.Chem. 7 (7), 1075 (1997). [25] S. I w a s a k i, Y. N a k a m u r a, IEEE Trans. Magn. MAG 13, 1272 (1977), w/g [18]. [26] T. O s a k a, N. N a g a s a k a, F. G o t o, J.Electrochem. Soc. 128, 1686 (1981). [27] H. M a s u d a, M. S a t o h, Jpn.J.Appl.Phys. 35, L 126 (1996). [28] D. G. W. G o a d, M. M o s k o v i t s, J. Appl. Phys. 49, 2929 (1978). Received: 10 February 2009.
16
O 2 ions are formed in the reaction:
 A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 59 2014 Issue 1 DOI: 10.2478/amm-2014-0022 W. GUMOWSKA, I. DOBOSZ, B. WRZOSZCZYK THE MORPHOLOGY OF THE ALUMINA FILMS FORMED IN THE
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 59 2014 Issue 1 DOI: 10.2478/amm-2014-0022 W. GUMOWSKA, I. DOBOSZ, B. WRZOSZCZYK THE MORPHOLOGY OF THE ALUMINA FILMS FORMED IN THE
INSPECTION METHODS FOR QUALITY CONTROL OF FIBRE METAL LAMINATES IN AEROSPACE COMPONENTS
 Kompozyty 11: 2 (2011) 130-135 Krzysztof Dragan 1 * Jarosław Bieniaś 2, Michał Sałaciński 1, Piotr Synaszko 1 1 Air Force Institute of Technology, Non Destructive Testing Lab., ul. ks. Bolesława 6, 01-494
Kompozyty 11: 2 (2011) 130-135 Krzysztof Dragan 1 * Jarosław Bieniaś 2, Michał Sałaciński 1, Piotr Synaszko 1 1 Air Force Institute of Technology, Non Destructive Testing Lab., ul. ks. Bolesława 6, 01-494
 www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume Issue 4
 A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 54 2009 Issue 4 D. LEŚNIAK STRUCTURE AND MECHANICAL PROPERTIES OF EXTRUDED AlCuMg SECTIONS IN T5 TEMPER STRUKTURA I WŁASNOŚCI MECHANICZNE
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 54 2009 Issue 4 D. LEŚNIAK STRUCTURE AND MECHANICAL PROPERTIES OF EXTRUDED AlCuMg SECTIONS IN T5 TEMPER STRUKTURA I WŁASNOŚCI MECHANICZNE
 www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
THERMODYNAMICS OF OXYGEN IN DILUTE LIQUID SILVER-TELLURIUM ALLOYS
 AGH University of Science and Technology Faculty of Non-Ferrous Metals Laboratory of Physical Chemistry and Electrochemistry THERMODYNAMICS OF OXYGEN IN DILUTE LIQUID SILVER-TELLURIUM ALLOYS Justyna Nyk,
AGH University of Science and Technology Faculty of Non-Ferrous Metals Laboratory of Physical Chemistry and Electrochemistry THERMODYNAMICS OF OXYGEN IN DILUTE LIQUID SILVER-TELLURIUM ALLOYS Justyna Nyk,
Dr inż. Paulina Indyka
 Dr inż. Paulina Indyka Kierownik pracy: dr hab. Ewa BełtowskaLehman, prof. PAN Tytuł pracy w języku polskim: Optymalizacja mikrostruktury i właściwości powłok NiW osadzanych elektrochemicznie Tytuł pracy
Dr inż. Paulina Indyka Kierownik pracy: dr hab. Ewa BełtowskaLehman, prof. PAN Tytuł pracy w języku polskim: Optymalizacja mikrostruktury i właściwości powłok NiW osadzanych elektrochemicznie Tytuł pracy
Development of SOFC technology in IEn OC Cerel
 Development of SOFC technology in IEn OC Cerel mgr. inż. Michał Kawalec dr inż. Ryszard Kluczowski dr inż. Mariusz Krauz ing. Jan Pieter Ouweltjes 1 Introduction 2 AS-SOFC technology 3 Cell tests 4 SOFC
Development of SOFC technology in IEn OC Cerel mgr. inż. Michał Kawalec dr inż. Ryszard Kluczowski dr inż. Mariusz Krauz ing. Jan Pieter Ouweltjes 1 Introduction 2 AS-SOFC technology 3 Cell tests 4 SOFC
DM-ML, DM-FL. Auxiliary Equipment and Accessories. Damper Drives. Dimensions. Descritpion
 DM-ML, DM-FL Descritpion DM-ML and DM-FL actuators are designed for driving round dampers and square multi-blade dampers. Example identification Product code: DM-FL-5-2 voltage Dimensions DM-ML-6 DM-ML-8
DM-ML, DM-FL Descritpion DM-ML and DM-FL actuators are designed for driving round dampers and square multi-blade dampers. Example identification Product code: DM-FL-5-2 voltage Dimensions DM-ML-6 DM-ML-8
 www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
ROZPRAWY NR 128. Stanis³aw Mroziñski
 UNIWERSYTET TECHNOLOGICZNO-PRZYRODNICZY IM. JANA I JÊDRZEJA ŒNIADECKICH W BYDGOSZCZY ROZPRAWY NR 128 Stanis³aw Mroziñski STABILIZACJA W ASNOŒCI CYKLICZNYCH METALI I JEJ WP YW NA TRWA OŒÆ ZMÊCZENIOW BYDGOSZCZ
UNIWERSYTET TECHNOLOGICZNO-PRZYRODNICZY IM. JANA I JÊDRZEJA ŒNIADECKICH W BYDGOSZCZY ROZPRAWY NR 128 Stanis³aw Mroziñski STABILIZACJA W ASNOŒCI CYKLICZNYCH METALI I JEJ WP YW NA TRWA OŒÆ ZMÊCZENIOW BYDGOSZCZ
Fig 5 Spectrograms of the original signal (top) extracted shaft-related GAD components (middle) and
 Fig 4 Measured vibration signal (top). Blue original signal. Red component related to periodic excitation of resonances and noise. Green component related. Rotational speed profile used for experiment
Fig 4 Measured vibration signal (top). Blue original signal. Red component related to periodic excitation of resonances and noise. Green component related. Rotational speed profile used for experiment
 www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
 !850016! www.irs.gov/form8879eo. e-file www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C,
!850016! www.irs.gov/form8879eo. e-file www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C,
G14L LPG toroidal tank
 G14L LPG toroidal tank Product Description 4-hole version of the unique STAKO patented design of the full (centre-filled) toroidal tank. The valve plate enhances the functionality of the tank compared
G14L LPG toroidal tank Product Description 4-hole version of the unique STAKO patented design of the full (centre-filled) toroidal tank. The valve plate enhances the functionality of the tank compared
Proposal of thesis topic for mgr in. (MSE) programme in Telecommunications and Computer Science
 Proposal of thesis topic for mgr in (MSE) programme 1 Topic: Monte Carlo Method used for a prognosis of a selected technological process 2 Supervisor: Dr in Małgorzata Langer 3 Auxiliary supervisor: 4
Proposal of thesis topic for mgr in (MSE) programme 1 Topic: Monte Carlo Method used for a prognosis of a selected technological process 2 Supervisor: Dr in Małgorzata Langer 3 Auxiliary supervisor: 4
TYRE PYROLYSIS. REDUXCO GENERAL DISTRIBUTOR :: ::
 TYRE PYROLYSIS Installation for rubber waste pyrolysis designed for processing of used tyres and plastic waste (polyethylene, polypropylene, polystyrene), where the final product could be electricity,
TYRE PYROLYSIS Installation for rubber waste pyrolysis designed for processing of used tyres and plastic waste (polyethylene, polypropylene, polystyrene), where the final product could be electricity,
Has the heat wave frequency or intensity changed in Poland since 1950?
 Has the heat wave frequency or intensity changed in Poland since 1950? Joanna Wibig Department of Meteorology and Climatology, University of Lodz, Poland OUTLINE: Motivation Data Heat wave frequency measures
Has the heat wave frequency or intensity changed in Poland since 1950? Joanna Wibig Department of Meteorology and Climatology, University of Lodz, Poland OUTLINE: Motivation Data Heat wave frequency measures
Machine Learning for Data Science (CS4786) Lecture11. Random Projections & Canonical Correlation Analysis
 Machine Learning for Data Science (CS4786) Lecture11 5 Random Projections & Canonical Correlation Analysis The Tall, THE FAT AND THE UGLY n X d The Tall, THE FAT AND THE UGLY d X > n X d n = n d d The
Machine Learning for Data Science (CS4786) Lecture11 5 Random Projections & Canonical Correlation Analysis The Tall, THE FAT AND THE UGLY n X d The Tall, THE FAT AND THE UGLY d X > n X d n = n d d The
Lecture 18 Review for Exam 1
 Spring, 2019 ME 323 Mechanics of Materials Lecture 18 Review for Exam 1 Reading assignment: HW1-HW5 News: Ready for the exam? Instructor: Prof. Marcial Gonzalez Announcements Exam 1 - Wednesday February
Spring, 2019 ME 323 Mechanics of Materials Lecture 18 Review for Exam 1 Reading assignment: HW1-HW5 News: Ready for the exam? Instructor: Prof. Marcial Gonzalez Announcements Exam 1 - Wednesday February
SHP / SHP-T Standard and Basic PLUS
 Range Features ErP compliant High Pressure Sodium Lamps Long life between 24,000 to 28,000 hours, T90 at 16,000 hours Strong performance with high reliability Car park, Street and Floodlighting applications
Range Features ErP compliant High Pressure Sodium Lamps Long life between 24,000 to 28,000 hours, T90 at 16,000 hours Strong performance with high reliability Car park, Street and Floodlighting applications
Stopy metali nieżelaznych Non-Ferrous Alloys
 MODULE DESCRIPTION Module code Module name Module name in English Valid from academic year 201/2014 MODULE PLACEMENT IN THE SYLLABUS Stopy metali nieżelaznych Non-Ferrous Alloys Subject Level of education
MODULE DESCRIPTION Module code Module name Module name in English Valid from academic year 201/2014 MODULE PLACEMENT IN THE SYLLABUS Stopy metali nieżelaznych Non-Ferrous Alloys Subject Level of education
STRUCTURE AND PROPERTIES OF Ni-P/PTFE COMPOSITE COATINGS PRODUCED BY CHEMICAL REDUCTION METHOD
 16: 3 (2016) 174-179 Maria Trzaska*, Grzegorz Cieślak, Anna Mazurek Institute of Precision Mechanics, ul. Duchnicka 3, 01-796 Warsaw, Poland *Corresponding author. E-mail: maria.trzaska@imp.edu.pl Received
16: 3 (2016) 174-179 Maria Trzaska*, Grzegorz Cieślak, Anna Mazurek Institute of Precision Mechanics, ul. Duchnicka 3, 01-796 Warsaw, Poland *Corresponding author. E-mail: maria.trzaska@imp.edu.pl Received
QUANTITATIVE AND QUALITATIVE CHARACTERISTICS OF FINGERPRINT BIOMETRIC TEMPLATES
 ZESZYTY NAUKOWE POLITECHNIKI ŚLĄSKIEJ 2014 Seria: ORGANIZACJA I ZARZĄDZANIE z. 74 Nr kol. 1921 Adrian KAPCZYŃSKI Politechnika Śląska Instytut Ekonomii i Informatyki QUANTITATIVE AND QUALITATIVE CHARACTERISTICS
ZESZYTY NAUKOWE POLITECHNIKI ŚLĄSKIEJ 2014 Seria: ORGANIZACJA I ZARZĄDZANIE z. 74 Nr kol. 1921 Adrian KAPCZYŃSKI Politechnika Śląska Instytut Ekonomii i Informatyki QUANTITATIVE AND QUALITATIVE CHARACTERISTICS
Streszczenie rozprawy doktorskiej
 Doskonalenie pomiaru zawartości wody w produktach spożywczych z wykorzystaniem metody wagosuszarkowej bazującej na promieniowaniu IR mgr Sławomir Janas Streszczenie rozprawy doktorskiej Promotor pracy:
Doskonalenie pomiaru zawartości wody w produktach spożywczych z wykorzystaniem metody wagosuszarkowej bazującej na promieniowaniu IR mgr Sławomir Janas Streszczenie rozprawy doktorskiej Promotor pracy:
DUAL SIMILARITY OF VOLTAGE TO CURRENT AND CURRENT TO VOLTAGE TRANSFER FUNCTION OF HYBRID ACTIVE TWO- PORTS WITH CONVERSION
 ELEKTRYKA 0 Zeszyt (9) Rok LX Andrzej KUKIEŁKA Politechnika Śląska w Gliwicach DUAL SIMILARITY OF VOLTAGE TO CURRENT AND CURRENT TO VOLTAGE TRANSFER FUNCTION OF HYBRID ACTIVE TWO- PORTS WITH CONVERSION
ELEKTRYKA 0 Zeszyt (9) Rok LX Andrzej KUKIEŁKA Politechnika Śląska w Gliwicach DUAL SIMILARITY OF VOLTAGE TO CURRENT AND CURRENT TO VOLTAGE TRANSFER FUNCTION OF HYBRID ACTIVE TWO- PORTS WITH CONVERSION
Patients price acceptance SELECTED FINDINGS
 Patients price acceptance SELECTED FINDINGS October 2015 Summary With growing economy and Poles benefiting from this growth, perception of prices changes - this is also true for pharmaceuticals It may
Patients price acceptance SELECTED FINDINGS October 2015 Summary With growing economy and Poles benefiting from this growth, perception of prices changes - this is also true for pharmaceuticals It may
Wpływ powłoki Al Si na proces wytwarzania i jakość zgrzewanych aluminiowanych rur stalowych
 Akademia Górniczo-Hutnicza im. Stanisława Staszica w Krakowie Wpływ powłoki Al Si na proces wytwarzania i jakość zgrzewanych aluminiowanych rur stalowych The influence of Al Si coating on the manufacturing
Akademia Górniczo-Hutnicza im. Stanisława Staszica w Krakowie Wpływ powłoki Al Si na proces wytwarzania i jakość zgrzewanych aluminiowanych rur stalowych The influence of Al Si coating on the manufacturing
Tytuł pracy w języku angielskim: Microstructural characterization of Ag/X/Ag (X = Sn, In) joints obtained as the effect of diffusion soledering.
 Dr inż. Przemysław Skrzyniarz Kierownik pracy: Prof. dr hab. inż. Paweł Zięba Tytuł pracy w języku polskim: Charakterystyka mikrostruktury spoin Ag/X/Ag (X = Sn, In) uzyskanych w wyniku niskotemperaturowego
Dr inż. Przemysław Skrzyniarz Kierownik pracy: Prof. dr hab. inż. Paweł Zięba Tytuł pracy w języku polskim: Charakterystyka mikrostruktury spoin Ag/X/Ag (X = Sn, In) uzyskanych w wyniku niskotemperaturowego
Przewody do linii napowietrznych Przewody z drutów okrągłych skręconych współosiowo
 POPRAWKA do POLSKIEJ NORMY ICS 29.060.10 PNEN 50182:2002/AC Wprowadza EN 50182:2001/AC:2013, IDT Przewody do linii napowietrznych Przewody z drutów okrągłych skręconych współosiowo Poprawka do Normy Europejskiej
POPRAWKA do POLSKIEJ NORMY ICS 29.060.10 PNEN 50182:2002/AC Wprowadza EN 50182:2001/AC:2013, IDT Przewody do linii napowietrznych Przewody z drutów okrągłych skręconych współosiowo Poprawka do Normy Europejskiej
Helena Boguta, klasa 8W, rok szkolny 2018/2019
 Poniższy zbiór zadań został wykonany w ramach projektu Mazowiecki program stypendialny dla uczniów szczególnie uzdolnionych - najlepsza inwestycja w człowieka w roku szkolnym 2018/2019. Składają się na
Poniższy zbiór zadań został wykonany w ramach projektu Mazowiecki program stypendialny dla uczniów szczególnie uzdolnionych - najlepsza inwestycja w człowieka w roku szkolnym 2018/2019. Składają się na
Institute of Metallurgy and Materials Science, Polish Academy of Sciences: metallurgist (since 2010), assistant professor (since 2014).
 Phone: (012) 295 28 12; (012) 295 28 22 (lab.); fax: (012) 295 28 04 email: h.kazimierczak@imim.pl Employment and positions Institute of Metallurgy and Materials Science, Polish Academy of Sciences: metallurgist
Phone: (012) 295 28 12; (012) 295 28 22 (lab.); fax: (012) 295 28 04 email: h.kazimierczak@imim.pl Employment and positions Institute of Metallurgy and Materials Science, Polish Academy of Sciences: metallurgist
BIOPHYSICS. Politechnika Łódzka, ul. Żeromskiego 116, Łódź, tel. (042)
 BIOPHYSICS Prezentacja multimedialna współfinansowana przez Unię Europejską w ramach Europejskiego Funduszu Społecznego w projekcie pt. Innowacyjna dydaktyka bez ograniczeń - zintegrowany rozwój Politechniki
BIOPHYSICS Prezentacja multimedialna współfinansowana przez Unię Europejską w ramach Europejskiego Funduszu Społecznego w projekcie pt. Innowacyjna dydaktyka bez ograniczeń - zintegrowany rozwój Politechniki
photo graphic Jan Witkowski Project for exhibition compositions typography colors : +48 506 780 943 : janwi@janwi.com
 Jan Witkowski : +48 506 780 943 : janwi@janwi.com Project for exhibition photo graphic compositions typography colors Berlin London Paris Barcelona Vienna Prague Krakow Zakopane Jan Witkowski ARTIST FROM
Jan Witkowski : +48 506 780 943 : janwi@janwi.com Project for exhibition photo graphic compositions typography colors Berlin London Paris Barcelona Vienna Prague Krakow Zakopane Jan Witkowski ARTIST FROM
Typ VME FOR THE MEASUREMENT OF VOLUME FLOW RATES IN DUCTS
 Typ VME FOR THE MEASUREMENT OF VOLUME FLOW RATES IN DUCTS Rectangular volume flow rate measuring units for the recording or monitoring of volume flow rates Manual volume flow rate measuring Permanent volume
Typ VME FOR THE MEASUREMENT OF VOLUME FLOW RATES IN DUCTS Rectangular volume flow rate measuring units for the recording or monitoring of volume flow rates Manual volume flow rate measuring Permanent volume
TECHNICAL CATALOGUE WHITEHEART MALLEABLE CAST IRON FITTINGS EE
 TECHNICAL CATALOGUE WHITEHEART MALLEABLE CAST IRON FITTINGS EE Poland GENERAL INFORMATION USE Whiteheart malleable cast iron fittings brand EE are used in threaded pipe joints, particularly in water, gas,
TECHNICAL CATALOGUE WHITEHEART MALLEABLE CAST IRON FITTINGS EE Poland GENERAL INFORMATION USE Whiteheart malleable cast iron fittings brand EE are used in threaded pipe joints, particularly in water, gas,
 www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes," complete Schedule C, Part
Typ VFR. Circular flow adjustment dampers for the adjustment of volume flow rates and pressures in supply air and extract air systems
 Typ VFR FOR THE RELIABLE BALANCING OF VOLUME FLOW RATES Circular flow adjustment dampers for the adjustment of volume flow rates and pressures in supply air and extract air systems Each flow adjustment
Typ VFR FOR THE RELIABLE BALANCING OF VOLUME FLOW RATES Circular flow adjustment dampers for the adjustment of volume flow rates and pressures in supply air and extract air systems Each flow adjustment
Investigation of the coexistence of superconductivity and magnetism in substituted EuFe 2 As 2. Lan Maria Tran
 Investigation of the coexistence of superconductivity and magnetism in substituted EuFe 2 As 2 Lan Maria Tran 27.06.2017, Wrocław ABSTRACT The recently discovered iron-based superconductors are one of
Investigation of the coexistence of superconductivity and magnetism in substituted EuFe 2 As 2 Lan Maria Tran 27.06.2017, Wrocław ABSTRACT The recently discovered iron-based superconductors are one of
Mikrostruktura, struktura magnetyczna oraz właściwości magnetyczne amorficznych i częściowo skrystalizowanych stopów Fe, Co i Ni
 mgr inż. Jakub Rzącki Praca doktorska p.t.: Mikrostruktura, struktura magnetyczna oraz właściwości magnetyczne amorficznych i częściowo skrystalizowanych stopów Fe, Co i Ni STRESZCZENIE W pracy przedstawiono
mgr inż. Jakub Rzącki Praca doktorska p.t.: Mikrostruktura, struktura magnetyczna oraz właściwości magnetyczne amorficznych i częściowo skrystalizowanych stopów Fe, Co i Ni STRESZCZENIE W pracy przedstawiono
Utilization of solutions obtained after magnesium removal from sphalerite concentrates with spent electrolyte derived from winning of cathode zinc
 GOSPODARKA SUROWCAMI MINERALNYMI Tom 29 2013 Zeszyt 4 DOI 10.2478/gospo-2013-0039 ANDRZEJ JAROSIÑSKI*, ADAM KOZAK**, SYLWESTER ELAZNY*** Utilization of solutions obtained after magnesium removal from sphalerite
GOSPODARKA SUROWCAMI MINERALNYMI Tom 29 2013 Zeszyt 4 DOI 10.2478/gospo-2013-0039 ANDRZEJ JAROSIÑSKI*, ADAM KOZAK**, SYLWESTER ELAZNY*** Utilization of solutions obtained after magnesium removal from sphalerite
ODPORNOŚĆ KOROZYJNA WARSTW KOMPOZYTOWYCH Z OSNOWĄ NIKLOWĄ I DYSPERSYJNĄ FAZĄ CERAMICZNĄ
 KOMPOZYTY (COMPOSITES) 2(2002)5 Maria Trzaska 1, Anna Wyszyńska 2, Magdalena Kowalewska 3 Politechnika Warszawska, Wydział Inżynierii Materiałowej, ul. Wołoska 141, 02-507 Warszawa ODPORNOŚĆ KOROZYJNA
KOMPOZYTY (COMPOSITES) 2(2002)5 Maria Trzaska 1, Anna Wyszyńska 2, Magdalena Kowalewska 3 Politechnika Warszawska, Wydział Inżynierii Materiałowej, ul. Wołoska 141, 02-507 Warszawa ODPORNOŚĆ KOROZYJNA
Tytuł pracy w języku angielskim: Physical properties of liquid crystal mixtures of chiral and achiral compounds for use in LCDs
 Dr inż. Jan Czerwiec Kierownik pracy: dr hab. Monika Marzec Tytuł pracy w języku polskim: Właściwości fizyczne mieszanin ciekłokrystalicznych związków chiralnych i achiralnych w odniesieniu do zastosowań
Dr inż. Jan Czerwiec Kierownik pracy: dr hab. Monika Marzec Tytuł pracy w języku polskim: Właściwości fizyczne mieszanin ciekłokrystalicznych związków chiralnych i achiralnych w odniesieniu do zastosowań
PROCEEDINGS OF THE INSTITUTE OF VEHICLES 2(106)/2016 (12 pt)
 PROCEEDINGS OF THE INSTITUTE OF VEHICLES 2(106)/2016 Maciej Foremny 1, Szymon Gudowski 2, Michał Malesza 3, Henryk Bąkowski 4 TRIBOLOGICAL WEAR ESTIMATION OF THE ENGINE OILS USED IN DRIFTING 1. Introduction
PROCEEDINGS OF THE INSTITUTE OF VEHICLES 2(106)/2016 Maciej Foremny 1, Szymon Gudowski 2, Michał Malesza 3, Henryk Bąkowski 4 TRIBOLOGICAL WEAR ESTIMATION OF THE ENGINE OILS USED IN DRIFTING 1. Introduction
EXAMPLES OF CABRI GEOMETRE II APPLICATION IN GEOMETRIC SCIENTIFIC RESEARCH
 Anna BŁACH Centre of Geometry and Engineering Graphics Silesian University of Technology in Gliwice EXAMPLES OF CABRI GEOMETRE II APPLICATION IN GEOMETRIC SCIENTIFIC RESEARCH Introduction Computer techniques
Anna BŁACH Centre of Geometry and Engineering Graphics Silesian University of Technology in Gliwice EXAMPLES OF CABRI GEOMETRE II APPLICATION IN GEOMETRIC SCIENTIFIC RESEARCH Introduction Computer techniques
 www.irs.gov/form8879eo. e-file www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes,"
www.irs.gov/form8879eo. e-file www.irs.gov/form990. If "Yes," complete Schedule A Schedule B, Schedule of Contributors If "Yes," complete Schedule C, Part I If "Yes," complete Schedule C, Part II If "Yes,"
WENTYLATORY PROMIENIOWE SINGLE-INLET DRUM BĘBNOWE JEDNOSTRUMIENIOWE CENTRIFUGAL FAN
 WENTYLATORY PROMIENIOWE SINGLE-INLET DRUM BĘBNOWE JEDNOSTRUMIENIOWE CENTRIFUGAL FAN TYP WPB TYPE WPB Wentylatory promieniowe jednostrumieniowe bębnowe (z wirnikiem typu Single-inlet centrifugal fans (with
WENTYLATORY PROMIENIOWE SINGLE-INLET DRUM BĘBNOWE JEDNOSTRUMIENIOWE CENTRIFUGAL FAN TYP WPB TYPE WPB Wentylatory promieniowe jednostrumieniowe bębnowe (z wirnikiem typu Single-inlet centrifugal fans (with
MATERIAŁY UŻYTE W BADANIACH
 4-2014 T R I B O L O G I A 9 Marek BARA * OCENA WPŁYWU PRZYGOTOWANIA PODŁOŻA NA WŁAŚCIWOŚCI TRIBOLOGICZNE NANOCERAMICZNYCH WARSTW TLENKOWYCH AN ESTIMATION OF THE INFLUENCE OF THE SUBSTRATE PREPARATION
4-2014 T R I B O L O G I A 9 Marek BARA * OCENA WPŁYWU PRZYGOTOWANIA PODŁOŻA NA WŁAŚCIWOŚCI TRIBOLOGICZNE NANOCERAMICZNYCH WARSTW TLENKOWYCH AN ESTIMATION OF THE INFLUENCE OF THE SUBSTRATE PREPARATION
Cracow University of Economics Poland. Overview. Sources of Real GDP per Capita Growth: Polish Regional-Macroeconomic Dimensions 2000-2005
 Cracow University of Economics Sources of Real GDP per Capita Growth: Polish Regional-Macroeconomic Dimensions 2000-2005 - Key Note Speech - Presented by: Dr. David Clowes The Growth Research Unit CE Europe
Cracow University of Economics Sources of Real GDP per Capita Growth: Polish Regional-Macroeconomic Dimensions 2000-2005 - Key Note Speech - Presented by: Dr. David Clowes The Growth Research Unit CE Europe
SSW1.1, HFW Fry #20, Zeno #25 Benchmark: Qtr.1. Fry #65, Zeno #67. like
 SSW1.1, HFW Fry #20, Zeno #25 Benchmark: Qtr.1 I SSW1.1, HFW Fry #65, Zeno #67 Benchmark: Qtr.1 like SSW1.2, HFW Fry #47, Zeno #59 Benchmark: Qtr.1 do SSW1.2, HFW Fry #5, Zeno #4 Benchmark: Qtr.1 to SSW1.2,
SSW1.1, HFW Fry #20, Zeno #25 Benchmark: Qtr.1 I SSW1.1, HFW Fry #65, Zeno #67 Benchmark: Qtr.1 like SSW1.2, HFW Fry #47, Zeno #59 Benchmark: Qtr.1 do SSW1.2, HFW Fry #5, Zeno #4 Benchmark: Qtr.1 to SSW1.2,
P/M COMPOSITES OF Al-Si-Fe-Cu ALLOY WITH SiC PARTICLES HOT-EXTRUDED AFTER PRELIMINARY COMPACTION
 Kompozyty 11: 4 (211) 336-341 Marek Wojtaszek AGH University of Science and Technology, Faculty of Metals Engineering and Industrial Computer Science, al. Mickiewicza 3, 3-59 Kraków, Poland * Corresponding
Kompozyty 11: 4 (211) 336-341 Marek Wojtaszek AGH University of Science and Technology, Faculty of Metals Engineering and Industrial Computer Science, al. Mickiewicza 3, 3-59 Kraków, Poland * Corresponding
Typ VFR. Circular flow adjustment dampers for the adjustment of volume flow rates and pressures in supply air and extract air systems
 Typ VFR FOR THE RELIABLE BALANCING OF VOLUME FLOW RATES Circular flow adjustment dampers for the adjustment of volume flow rates and pressures in supply air and extract air systems Each flow adjustment
Typ VFR FOR THE RELIABLE BALANCING OF VOLUME FLOW RATES Circular flow adjustment dampers for the adjustment of volume flow rates and pressures in supply air and extract air systems Each flow adjustment
ROZPRAWA DOKTORSKA. Model obliczeniowy ogrzewań mikroprzewodowych
 POLITECHNIKA WARSZAWSKA Wydział Inżynierii Środowiska Instytut Ogrzewnictwa i Wentylacji ROZPRAWA DOKTORSKA mgr inż. Michał Strzeszewski Model obliczeniowy ogrzewań mikroprzewodowych (streszczenie) Promotor
POLITECHNIKA WARSZAWSKA Wydział Inżynierii Środowiska Instytut Ogrzewnictwa i Wentylacji ROZPRAWA DOKTORSKA mgr inż. Michał Strzeszewski Model obliczeniowy ogrzewań mikroprzewodowych (streszczenie) Promotor
Krytyczne czynniki sukcesu w zarządzaniu projektami
 Seweryn SPAŁEK Krytyczne czynniki sukcesu w zarządzaniu projektami MONOGRAFIA Wydawnictwo Politechniki Śląskiej Gliwice 2004 SPIS TREŚCI WPROWADZENIE 5 1. ZARZĄDZANIE PROJEKTAMI W ORGANIZACJI 13 1.1. Zarządzanie
Seweryn SPAŁEK Krytyczne czynniki sukcesu w zarządzaniu projektami MONOGRAFIA Wydawnictwo Politechniki Śląskiej Gliwice 2004 SPIS TREŚCI WPROWADZENIE 5 1. ZARZĄDZANIE PROJEKTAMI W ORGANIZACJI 13 1.1. Zarządzanie
Sargent Opens Sonairte Farmers' Market
 Sargent Opens Sonairte Farmers' Market 31 March, 2008 1V8VIZSV7EVKIRX8(1MRMWXIVSJ7XEXIEXXLI(ITEVXQIRXSJ%KVMGYPXYVI *MWLIVMIWERH*SSHTIVJSVQIHXLISJJMGMEPSTIRMRKSJXLI7SREMVXI*EVQIVW 1EVOIXMR0E]XS[R'S1IEXL
Sargent Opens Sonairte Farmers' Market 31 March, 2008 1V8VIZSV7EVKIRX8(1MRMWXIVSJ7XEXIEXXLI(ITEVXQIRXSJ%KVMGYPXYVI *MWLIVMIWERH*SSHTIVJSVQIHXLISJJMGMEPSTIRMRKSJXLI7SREMVXI*EVQIVW 1EVOIXMR0E]XS[R'S1IEXL
Formation of Graded Structures and Properties in Metal Matrix Composites by use of Electromagnetic Field
 Ludmil Drenchev Jerzy J. Sobczak Formation of Graded Structures and Properties in Metal Matrix Composites by use of Electromagnetic Field This project is funded by the Ministry of Education, Youth and
Ludmil Drenchev Jerzy J. Sobczak Formation of Graded Structures and Properties in Metal Matrix Composites by use of Electromagnetic Field This project is funded by the Ministry of Education, Youth and
Institutional Determinants of IncomeLevel Convergence in the European. Union: Are Institutions Responsible for Divergence Tendencies of Some
 Institutional Determinants of IncomeLevel Convergence in the European Union: Are Institutions Responsible for Divergence Tendencies of Some Countries? Dr Mariusz Próchniak Katedra Ekonomii II, Szkoła Główna
Institutional Determinants of IncomeLevel Convergence in the European Union: Are Institutions Responsible for Divergence Tendencies of Some Countries? Dr Mariusz Próchniak Katedra Ekonomii II, Szkoła Główna
The impact of the global gravity field models on the orbit determination of LAGEOS satellites
 models on the Satelitarne metody wyznaczania pozycji we współczesnej geodezji i nawigacji, Poland 2-4.06.2011 Krzysztof Sośnica, Daniela Thaller, Adrian Jäggi, Rolf Dach and Gerhard Beutler Astronomical
models on the Satelitarne metody wyznaczania pozycji we współczesnej geodezji i nawigacji, Poland 2-4.06.2011 Krzysztof Sośnica, Daniela Thaller, Adrian Jäggi, Rolf Dach and Gerhard Beutler Astronomical
TRANSPORT W RODZINNYCH GOSPODARSTWACH ROLNYCH
 INŻYNIERIA W ROLNICTWIE. MONOGRAFIE 16 ENGINEERING IN AGRICULTURE. MONOGRAPHS 16 WIESŁAW GOLKA TRANSPORT W RODZINNYCH GOSPODARSTWACH ROLNYCH TRANSPORTATION IN RURAL FAMILY FARMS Falenty 2014 WYDAWNICTWO
INŻYNIERIA W ROLNICTWIE. MONOGRAFIE 16 ENGINEERING IN AGRICULTURE. MONOGRAPHS 16 WIESŁAW GOLKA TRANSPORT W RODZINNYCH GOSPODARSTWACH ROLNYCH TRANSPORTATION IN RURAL FAMILY FARMS Falenty 2014 WYDAWNICTWO
Machine Learning for Data Science (CS4786) Lecture 11. Spectral Embedding + Clustering
 Machine Learning for Data Science (CS4786) Lecture 11 Spectral Embedding + Clustering MOTIVATING EXAMPLE What can you say from this network? MOTIVATING EXAMPLE How about now? THOUGHT EXPERIMENT For each
Machine Learning for Data Science (CS4786) Lecture 11 Spectral Embedding + Clustering MOTIVATING EXAMPLE What can you say from this network? MOTIVATING EXAMPLE How about now? THOUGHT EXPERIMENT For each
Installation of EuroCert software for qualified electronic signature
 Installation of EuroCert software for qualified electronic signature for Microsoft Windows systems Warsaw 28.08.2019 Content 1. Downloading and running the software for the e-signature... 3 a) Installer
Installation of EuroCert software for qualified electronic signature for Microsoft Windows systems Warsaw 28.08.2019 Content 1. Downloading and running the software for the e-signature... 3 a) Installer
Typ MFPCR FOR THE MOST DEMANDING REQUIREMENTS ON THE PURITY OF INDOOR AIR, WORKSTATIONS, AND DEVICES
 Typ MFPCR FOR THE MOST DEMANDING REQUIREMENTS ON THE PURITY OF INDOOR AIR, WORKSTATIONS, AND DEVICES HEPA and ULPA filters as high-efficiency particulate filters for the separation of suspended particles
Typ MFPCR FOR THE MOST DEMANDING REQUIREMENTS ON THE PURITY OF INDOOR AIR, WORKSTATIONS, AND DEVICES HEPA and ULPA filters as high-efficiency particulate filters for the separation of suspended particles
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume Issue 1 DOI: /v y
 A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 57 2012 Issue 1 DOI: 10.2478/v10172-011-0149-y G. BOCZKAL, P. MARECKI, M. PEREK-NOWAK THE INFLUENCE OF SOLDERING CONDITIONS ON CONDUCTIVITY,
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 57 2012 Issue 1 DOI: 10.2478/v10172-011-0149-y G. BOCZKAL, P. MARECKI, M. PEREK-NOWAK THE INFLUENCE OF SOLDERING CONDITIONS ON CONDUCTIVITY,
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume Issue 2 DOI: /amm
 A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 60 2015 Issue 2 DOI: 10.1515/amm-2015-0200 K. LANKIEWICZ,, M. BARANOWSKI, T. BABUL, S. KOWALSKI THE STUDY OF THE IMPACT OF SURFACE
A R C H I V E S O F M E T A L L U R G Y A N D M A T E R I A L S Volume 60 2015 Issue 2 DOI: 10.1515/amm-2015-0200 K. LANKIEWICZ,, M. BARANOWSKI, T. BABUL, S. KOWALSKI THE STUDY OF THE IMPACT OF SURFACE
WYKAZ PRÓB / SUMMARY OF TESTS. mgr ing. Janusz Bandel
 Sprawozdanie z Badań Nr Strona/Page 2/24 WYKAZ PRÓB / SUMMARY OF TESTS STRONA PAGE Próba uszkodzenia przy przepięciach dorywczych TOV failure test 5 Próby wykonał / The tests were carried out by: mgr ing.
Sprawozdanie z Badań Nr Strona/Page 2/24 WYKAZ PRÓB / SUMMARY OF TESTS STRONA PAGE Próba uszkodzenia przy przepięciach dorywczych TOV failure test 5 Próby wykonał / The tests were carried out by: mgr ing.
TACHOGRAPH SIMULATOR DTCOSIM
 TACHOGRAPH SIMULATOR DTCOSIM Service Manual USB-KSIM interface General description The simulator is a device that is used as a replacement for tachograph in the vehicle where the tachograph is not mandatory,
TACHOGRAPH SIMULATOR DTCOSIM Service Manual USB-KSIM interface General description The simulator is a device that is used as a replacement for tachograph in the vehicle where the tachograph is not mandatory,
DOI: / /32/37
 . 2015. 4 (32) 1:18 DOI: 10.17223/1998863 /32/37 -,,. - -. :,,,,., -, -.,.-.,.,.,. -., -,.,,., -, 70 80. (.,.,. ),, -,.,, -,, (1886 1980).,.,, (.,.,..), -, -,,,, ; -, - 346, -,.. :, -, -,,,,,.,,, -,,,
. 2015. 4 (32) 1:18 DOI: 10.17223/1998863 /32/37 -,,. - -. :,,,,., -, -.,.-.,.,.,. -., -,.,,., -, 70 80. (.,.,. ),, -,.,, -,, (1886 1980).,.,, (.,.,..), -, -,,,, ; -, - 346, -,.. :, -, -,,,,,.,,, -,,,
Inquiry Form for Magnets
 Inquiry Form for Magnets Required scope of delivery: Yes No - Cross-beams - Magnets - Supply and Control System - Emergency supply system, backup time min - Cable drum with cable - Plug-in connections
Inquiry Form for Magnets Required scope of delivery: Yes No - Cross-beams - Magnets - Supply and Control System - Emergency supply system, backup time min - Cable drum with cable - Plug-in connections
BARIERA ANTYKONDENSACYJNA
 Skład Obróbka Parametry techniczne BARIERA ANTYKONDENSACYJNA Lama "Lama" sp. z o.o. sp. k Właściwość Metoda badania Wartość Jednostka włóknina poliestrowa + klej PSA + folia polietylenowa Samoprzylepna
Skład Obróbka Parametry techniczne BARIERA ANTYKONDENSACYJNA Lama "Lama" sp. z o.o. sp. k Właściwość Metoda badania Wartość Jednostka włóknina poliestrowa + klej PSA + folia polietylenowa Samoprzylepna
Tomasz KMITA Władysław SKONECZNY. 1. Wprowadzenie. 1. Introduction
 Tomasz KMITA Władysław SKONECZNY Wzrost trwałości eksploatacyjnej skojarzenia tworzywo polimerowe - powłoka tlenkowa wytwarzana metodą anodowania impulsowego Increase of operational durability of a plastic
Tomasz KMITA Władysław SKONECZNY Wzrost trwałości eksploatacyjnej skojarzenia tworzywo polimerowe - powłoka tlenkowa wytwarzana metodą anodowania impulsowego Increase of operational durability of a plastic
Few-fermion thermometry
 Few-fermion thermometry Phys. Rev. A 97, 063619 (2018) Tomasz Sowiński Institute of Physics of the Polish Academy of Sciences Co-authors: Marcin Płodzień Rafał Demkowicz-Dobrzański FEW-BODY PROBLEMS FewBody.ifpan.edu.pl
Few-fermion thermometry Phys. Rev. A 97, 063619 (2018) Tomasz Sowiński Institute of Physics of the Polish Academy of Sciences Co-authors: Marcin Płodzień Rafał Demkowicz-Dobrzański FEW-BODY PROBLEMS FewBody.ifpan.edu.pl
UPORZĄDKOWANE NANOSTRUKTURY UZYSKIWANE NA DRODZE ANODYZACJI ALUMINIUM
 Jagiellonian University Department of Physical Chemistry & Electrochemistry Ingardena 3, 30-060 060 Krakow, Poland UPORZĄDKOWANE NANOSTRUKTURY UZYSKIWANE NA DRODZE ANODYZACJI ALUMINIUM Grzegorz D. Sulka
Jagiellonian University Department of Physical Chemistry & Electrochemistry Ingardena 3, 30-060 060 Krakow, Poland UPORZĄDKOWANE NANOSTRUKTURY UZYSKIWANE NA DRODZE ANODYZACJI ALUMINIUM Grzegorz D. Sulka
Auditorium classes. Lectures
 Faculty of: Mechanical and Robotics Field of study: Mechatronic with English as instruction language Study level: First-cycle studies Form and type of study: Full-time studies Annual: 2016/2017 Lecture
Faculty of: Mechanical and Robotics Field of study: Mechatronic with English as instruction language Study level: First-cycle studies Form and type of study: Full-time studies Annual: 2016/2017 Lecture
Rev Źródło:
 KAmduino UNO Rev. 20190119182847 Źródło: http://wiki.kamamilabs.com/index.php/kamduino_uno Spis treści Basic features and parameters... 1 Standard equipment... 2 Electrical schematics... 3 AVR ATmega328P
KAmduino UNO Rev. 20190119182847 Źródło: http://wiki.kamamilabs.com/index.php/kamduino_uno Spis treści Basic features and parameters... 1 Standard equipment... 2 Electrical schematics... 3 AVR ATmega328P
OpenPoland.net API Documentation
 OpenPoland.net API Documentation Release 1.0 Michał Gryczka July 11, 2014 Contents 1 REST API tokens: 3 1.1 How to get a token............................................ 3 2 REST API : search for assets
OpenPoland.net API Documentation Release 1.0 Michał Gryczka July 11, 2014 Contents 1 REST API tokens: 3 1.1 How to get a token............................................ 3 2 REST API : search for assets
PRZEWODNIK PO PRZEDMIOCIE. Negotiation techniques. Management. Stationary. II degree
 Politechnika Częstochowska, Wydział Zarządzania PRZEWODNIK PO PRZEDMIOCIE Nazwa przedmiotu Kierunek Forma studiów Poziom kwalifikacji Rok Semestr Jednostka prowadząca Osoba sporządzająca Profil Rodzaj
Politechnika Częstochowska, Wydział Zarządzania PRZEWODNIK PO PRZEDMIOCIE Nazwa przedmiotu Kierunek Forma studiów Poziom kwalifikacji Rok Semestr Jednostka prowadząca Osoba sporządzająca Profil Rodzaj
Pro-tumoral immune cell alterations in wild type and Shbdeficient mice in response to 4T1 breast carcinomas
 www.oncotarget.com Oncotarget, Supplementary Materials Pro-tumoral immune cell alterations in wild type and Shbdeficient mice in response to 4T1 breast carcinomas SUPPLEMENTARY MATERIALS Supplementary
www.oncotarget.com Oncotarget, Supplementary Materials Pro-tumoral immune cell alterations in wild type and Shbdeficient mice in response to 4T1 breast carcinomas SUPPLEMENTARY MATERIALS Supplementary
Knovel Math: Jakość produktu
 Knovel Math: Jakość produktu Knovel jest agregatorem materiałów pełnotekstowych dostępnych w formacie PDF i interaktywnym. Narzędzia interaktywne Knovel nie są stworzone wokół specjalnych algorytmów wymagających
Knovel Math: Jakość produktu Knovel jest agregatorem materiałów pełnotekstowych dostępnych w formacie PDF i interaktywnym. Narzędzia interaktywne Knovel nie są stworzone wokół specjalnych algorytmów wymagających
Łukasz Reszka Wiceprezes Zarządu
 Łukasz Reszka Wiceprezes Zarządu Time for changes! Vocational activisation young unemployed people aged 15 to 24 Projekt location Ząbkowice Śląskie project produced in cooperation with Poviat Labour Office
Łukasz Reszka Wiceprezes Zarządu Time for changes! Vocational activisation young unemployed people aged 15 to 24 Projekt location Ząbkowice Śląskie project produced in cooperation with Poviat Labour Office
Streszczenia / Abstracts 2 / 2014
 Anna Olbrycht Powłoki metalowe jako zabezpieczenie antykorozyjne konstrukcji stalowych Metal coatings as corrosion protection of steel structures W artykule przedstawiono cele i zasady nakładania powłok
Anna Olbrycht Powłoki metalowe jako zabezpieczenie antykorozyjne konstrukcji stalowych Metal coatings as corrosion protection of steel structures W artykule przedstawiono cele i zasady nakładania powłok
DETECTION OF MATERIAL INTEGRATED CONDUCTORS FOR CONNECTIVE RIVETING OF FUNCTION-INTEGRATIVE TEXTILE-REINFORCED THERMOPLASTIC COMPOSITES
 Kompozyty 11: 2 (2011) 152-156 Werner A. Hufenbach, Frank Adam, Maik Gude, Ivonne Körner, Thomas Heber*, Anja Winkler Technische Universität Dresden, Institute of Lightweight Engineering and Polymer Technology
Kompozyty 11: 2 (2011) 152-156 Werner A. Hufenbach, Frank Adam, Maik Gude, Ivonne Körner, Thomas Heber*, Anja Winkler Technische Universität Dresden, Institute of Lightweight Engineering and Polymer Technology
WPŁYW PARAMETRÓW PROCESU ANODOWANIA IMPULSOWEGO NA TOPOGRAFIĘ POWIERZCHNI ANODOWYCH POWŁOK TLENKOWYCH NA ALUMINIUM
 3-2012 T R I B O L O G I A 61 Tomasz KMITA * WPŁYW PARAMETRÓW PROCESU ANODOWANIA IMPULSOWEGO NA TOPOGRAFIĘ POWIERZCHNI ANODOWYCH POWŁOK TLENKOWYCH NA ALUMINIUM THE INFLUENCE OF THE PULSED ANODISING PROCESS
3-2012 T R I B O L O G I A 61 Tomasz KMITA * WPŁYW PARAMETRÓW PROCESU ANODOWANIA IMPULSOWEGO NA TOPOGRAFIĘ POWIERZCHNI ANODOWYCH POWŁOK TLENKOWYCH NA ALUMINIUM THE INFLUENCE OF THE PULSED ANODISING PROCESS
Rozpoznawanie twarzy metodą PCA Michał Bereta 1. Testowanie statystycznej istotności różnic między jakością klasyfikatorów
 Rozpoznawanie twarzy metodą PCA Michał Bereta www.michalbereta.pl 1. Testowanie statystycznej istotności różnic między jakością klasyfikatorów Wiemy, że możemy porównywad klasyfikatory np. za pomocą kroswalidacji.
Rozpoznawanie twarzy metodą PCA Michał Bereta www.michalbereta.pl 1. Testowanie statystycznej istotności różnic między jakością klasyfikatorów Wiemy, że możemy porównywad klasyfikatory np. za pomocą kroswalidacji.
Technika jonowego rozpylenia
 Technika jonowego rozpylenia dr K.Marszałek 1 Technika jonowego rozpylenia dr K.Marszałek 2 Mechanizm rozpylenia dr K.Marszałek 3 :Création et transferet des différentes espèces en pulvérisation cathodique
Technika jonowego rozpylenia dr K.Marszałek 1 Technika jonowego rozpylenia dr K.Marszałek 2 Mechanizm rozpylenia dr K.Marszałek 3 :Création et transferet des différentes espèces en pulvérisation cathodique
BADANIA ELEKTROMAGNESÓW NADPRZEWODNIKOWYCH W PROCESIE ICH WYTWARZANIA I EKSPLOATACJI
 INSTYTUT ELEKTROTECHNIKI Janusz KOZAK BADANIA ELEKTROMAGNESÓW NADPRZEWODNIKOWYCH W PROCESIE ICH WYTWARZANIA I EKSPLOATACJI Prace Instytutu Elektrotechniki zeszyt 265, 2014 SPIS TRE CI STRESZCZENIE... 9
INSTYTUT ELEKTROTECHNIKI Janusz KOZAK BADANIA ELEKTROMAGNESÓW NADPRZEWODNIKOWYCH W PROCESIE ICH WYTWARZANIA I EKSPLOATACJI Prace Instytutu Elektrotechniki zeszyt 265, 2014 SPIS TRE CI STRESZCZENIE... 9
Akademia Morska w Szczecinie. Wydział Mechaniczny
 Akademia Morska w Szczecinie Wydział Mechaniczny ROZPRAWA DOKTORSKA mgr inż. Marcin Kołodziejski Analiza metody obsługiwania zarządzanego niezawodnością pędników azymutalnych platformy pływającej Promotor:
Akademia Morska w Szczecinie Wydział Mechaniczny ROZPRAWA DOKTORSKA mgr inż. Marcin Kołodziejski Analiza metody obsługiwania zarządzanego niezawodnością pędników azymutalnych platformy pływającej Promotor:
Fixtures LED HEDRION
 K A R T Y K ATA L O G O W E Fixtures LED HEDRION Oprawy lampy LED Hedrion do zastosowań profesjonalnych Fixtures LED lamps Hedrion for professional applications NATRIUM Sp. z o.o. ul. Grodziska 15, 05-870
K A R T Y K ATA L O G O W E Fixtures LED HEDRION Oprawy lampy LED Hedrion do zastosowań profesjonalnych Fixtures LED lamps Hedrion for professional applications NATRIUM Sp. z o.o. ul. Grodziska 15, 05-870
Nazwa projektu: Kreatywni i innowacyjni uczniowie konkurencyjni na rynku pracy
 Nazwa projektu: Kreatywni i innowacyjni uczniowie konkurencyjni na rynku pracy DZIAŁANIE 3.2 EDUKACJA OGÓLNA PODDZIAŁANIE 3.2.1 JAKOŚĆ EDUKACJI OGÓLNEJ Projekt współfinansowany przez Unię Europejską w
Nazwa projektu: Kreatywni i innowacyjni uczniowie konkurencyjni na rynku pracy DZIAŁANIE 3.2 EDUKACJA OGÓLNA PODDZIAŁANIE 3.2.1 JAKOŚĆ EDUKACJI OGÓLNEJ Projekt współfinansowany przez Unię Europejską w
Weronika Mysliwiec, klasa 8W, rok szkolny 2018/2019
 Poniższy zbiór zadań został wykonany w ramach projektu Mazowiecki program stypendialny dla uczniów szczególnie uzdolnionych - najlepsza inwestycja w człowieka w roku szkolnym 2018/2019. Tresci zadań rozwiązanych
Poniższy zbiór zadań został wykonany w ramach projektu Mazowiecki program stypendialny dla uczniów szczególnie uzdolnionych - najlepsza inwestycja w człowieka w roku szkolnym 2018/2019. Tresci zadań rozwiązanych
Zarządzanie sieciami telekomunikacyjnymi
 SNMP Protocol The Simple Network Management Protocol (SNMP) is an application layer protocol that facilitates the exchange of management information between network devices. It is part of the Transmission
SNMP Protocol The Simple Network Management Protocol (SNMP) is an application layer protocol that facilitates the exchange of management information between network devices. It is part of the Transmission
DELTIM Sp. z o.o. S.K.A ul. Rząsawska 30/38; Częstochowa. Bumper bar X-Lander X-Move
 Strona Page: 1 Zleceniodawca: Client: DELTIM Sp. z o.o. S.K.A ul. Rząsawska 30/38; 42-209 Częstochowa Przedmiot badania: Test item: Bumper bar X-Lander X-Move Producent / Klient zew.: Manufacturer / ext.
Strona Page: 1 Zleceniodawca: Client: DELTIM Sp. z o.o. S.K.A ul. Rząsawska 30/38; 42-209 Częstochowa Przedmiot badania: Test item: Bumper bar X-Lander X-Move Producent / Klient zew.: Manufacturer / ext.
W trzech niezależnych testach frezy z powłoką X tremeblue typu V803 był w każdym przypadku prawie 2 razy bardziej wydajne niż wersja niepowlekana.
 To nowa powłoka ochronna i jest znacznie lepsza jak DLC, - X-TremeBLUE jest nową aplikacją powlekania oparta na najnowszych technologiach NANO struktury. - X-TremeBLUE to powłoka o mikronowej grubości,
To nowa powłoka ochronna i jest znacznie lepsza jak DLC, - X-TremeBLUE jest nową aplikacją powlekania oparta na najnowszych technologiach NANO struktury. - X-TremeBLUE to powłoka o mikronowej grubości,
ANALYSIS OF VOLUME CHANGES OF SELECTED CEREAL GROUND GRAIN IN RESULT OF LOADING*
 Agricultural Engineering 7(132)/2011 ANALYSIS OF VOLUME CHANGES OF SELECTED CEREAL GROUND GRAIN IN RESULT OF LOADING* Rafał Nadulski, Elżbieta Kusińska, Zbigniew Kobus, Tomasz Guz, Zbigniew Oszczak Department
Agricultural Engineering 7(132)/2011 ANALYSIS OF VOLUME CHANGES OF SELECTED CEREAL GROUND GRAIN IN RESULT OF LOADING* Rafał Nadulski, Elżbieta Kusińska, Zbigniew Kobus, Tomasz Guz, Zbigniew Oszczak Department
PARAMETRY TECHNICZNE DEKLAROWANE PRZEZ PRODUCENTA POTWIERDZONE BADANIAMI / RATINGS ASSIGNED BY THE MANUFACTURER AND PROVED BY TESTS
 Sprawozdanie z Strona/Page 2/24 PARAMETRY TECHNICZNE DEKLAROWANE PRZEZ PRODUCENTA POTWIERDZONE BADANIAMI / RATINGS ASSIGNED BY THE MANUFACTURER AND PROVED BY TESTS Typ Type Napięcie trwałej pracy Continuous
Sprawozdanie z Strona/Page 2/24 PARAMETRY TECHNICZNE DEKLAROWANE PRZEZ PRODUCENTA POTWIERDZONE BADANIAMI / RATINGS ASSIGNED BY THE MANUFACTURER AND PROVED BY TESTS Typ Type Napięcie trwałej pracy Continuous
THE EFFECT OF THE METHOD OF POURING LIQUID METAL INTO MOULD ON MISRUN FORMATION IN THE CASTINGS MADE WITH LOST-WAX CASTING TECHNOLOGY
 A R C H I V E S O F M E C H A N I C A L T E C H N O L O G Y A N D A U T O M A T I O N Vol. 33 no. 4 2013 KRZYSZTOF GRZE KOWIAK, MARCIN WOJCIECHOWSKI KAMIL SYTEK THE EFFECT OF THE METHOD OF POURING LIQUID
A R C H I V E S O F M E C H A N I C A L T E C H N O L O G Y A N D A U T O M A T I O N Vol. 33 no. 4 2013 KRZYSZTOF GRZE KOWIAK, MARCIN WOJCIECHOWSKI KAMIL SYTEK THE EFFECT OF THE METHOD OF POURING LIQUID
OTRZYMYWANIE KOMPOZYTÓW METALOWO-CERAMICZNYCH METODAMI PLAZMOWYMI
 KOMPOZYTY (COMPOSITES) 1(21)1 Władysław Włosiński 1, Tomasz Chmielewski 2 Politechnika Warszawska, Instytut Technologii Materiałowych, ul. Narbutta 85, 2-542 Warszawa OTRZYMYWANIE KOMPOZYTÓW METALOWO-CERAMICZNYCH
KOMPOZYTY (COMPOSITES) 1(21)1 Władysław Włosiński 1, Tomasz Chmielewski 2 Politechnika Warszawska, Instytut Technologii Materiałowych, ul. Narbutta 85, 2-542 Warszawa OTRZYMYWANIE KOMPOZYTÓW METALOWO-CERAMICZNYCH
Microsystems in Medical Applications Liquid Flow Sensors
 Microsystems in Medical Applications Liquid Flow Sensors Prezentacja multimedialna współfinansowana przez Unię Europejską w ramach Europejskiego Funduszu Społecznego w projekcie pt. Innowacyjna dydaktyka
Microsystems in Medical Applications Liquid Flow Sensors Prezentacja multimedialna współfinansowana przez Unię Europejską w ramach Europejskiego Funduszu Społecznego w projekcie pt. Innowacyjna dydaktyka
Metodyki projektowania i modelowania systemów Cyganek & Kasperek & Rajda 2013 Katedra Elektroniki AGH
 Kierunek Elektronika i Telekomunikacja, Studia II stopnia Specjalność: Systemy wbudowane Metodyki projektowania i modelowania systemów Cyganek & Kasperek & Rajda 2013 Katedra Elektroniki AGH Zagadnienia
Kierunek Elektronika i Telekomunikacja, Studia II stopnia Specjalność: Systemy wbudowane Metodyki projektowania i modelowania systemów Cyganek & Kasperek & Rajda 2013 Katedra Elektroniki AGH Zagadnienia
MODIFYING THE STRUCTURE OF CERTAIN STEEL GRADES BY LOW-TEMPERATURE GLOW DISCHARGE ASSISTED NITRIDING
 4-2006 MAINTENANCE PROBLEMS 197 Janusz TROJANOWSKI, Institute of Precision Mechanics, Warsaw Ryszard SITEK, Tadeusz WIERZCHOŃ Warsaw University of Technology, Warsaw MODIFYING THE STRUCTURE OF CERTAIN
4-2006 MAINTENANCE PROBLEMS 197 Janusz TROJANOWSKI, Institute of Precision Mechanics, Warsaw Ryszard SITEK, Tadeusz WIERZCHOŃ Warsaw University of Technology, Warsaw MODIFYING THE STRUCTURE OF CERTAIN
